Abstract
We developed an intelligent device capable of controlled release of anti-cancer drugs. The nano-carrier consists of two key components: firstly, magnetic nanoparticles were synthesized through co-precipitation and then functionalized for attaching anti-cancer drugs; secondly, a polymer (poly(N-isopropylacrylamide-co-acrylic acid)) sensitive to pH and temperature was synthesized and employed to encapsulate the drug-loaded magnetic nanoparticles. This responsive polymer exhibits a lower critical solution temperature (LCST) of 38.5 °C, indicating a phase transition behavior. Numerous techniques and analyses, including Fourier transform infrared spectroscopy, thermogravimetric analysis, zeta potentials, scanning electron microscope, and transmission electron microscopy, were employed to confirm the successful execution of the aforementioned process. In vitro release assessments of the anti-cancer drug, doxorubicin, were conducted across various media (pH 5–8 and temperatures ranging from 20 to 40 °C). The outcomes revealed higher drug releases at temperatures exceeding the LCST (40 °C) and at slightly acidic pH levels (5–5.3). Notably, compared to the effectiveness of the unloaded drug, the magnetic smart polymer loaded with DOX exhibited a more potent cytotoxic effect. Considering these results, this nano-carrier emerges as a promising candidate for targeted therapeutic delivery to cancerous tissues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recent years have witnessed significant attention directed toward exploring the applications of biomolecules in conjunction with nanomaterials [1,2,3]. Concurrently, a novel targeted drug delivery system has emerged, designed to finely regulate release rates precisely when needed. These advancements are geared toward mitigating, if not eradicating, the undesirable side effects associated with such treatments [4,5,6,7].
Recently, an intensified interest has arisen in utilizing superparamagnetic nanoparticles as magnetic vehicles for diverse applications including hyperthermia [8,9,10], controlled drug release [11,12,13], and as contrasting agents for MRI [14, 15]. These nanoparticles have the capacity to responsively react to time-dependent fluctuations in magnetic fields, harnessing energy from these changes to induce temperature elevation. This intrinsic characteristic renders nanoparticles valuable in hyperthermia therapies and as activating agents in chemotherapy and radiotherapy.
Ferrite manganese nanoparticles have surfaced as the foremost contenders in this domain. Their noteworthy attributes such as non-toxicity, substantial biocompatibility, and adeptness in simultaneous magnetic field manipulation make them well-suited for medical applications.
Recent studies have revealed that externally applied magnetic fields can serve as guides, directing surface-modified superparamagnetic nanoparticles to their intended targets [16]. However, the absence of surface modifications can lead to the aggregation and formation of clusters, causing the loss of superparamagnetic properties in nanoparticles.
One of the limitations associated with the utilization of magnetic nanoparticles in drug delivery systems is their tendency to be cleared by reticuloendothelial system (RES) macrophages before reaching their desired tumor cells [17]. Therefore, it becomes imperative to introduce water-soluble functional groups onto the surfaces of drug-loaded nanoparticles to enable their biomedical application.
To address these challenges and to minimize the adverse effects of established anti-cancer drugs, the preference lies in employing composite nanoparticles featuring a functionalized magnetic core encased within a biodegradable polymer shell [18, 19]. This choice is driven by the reasons mentioned earlier and underscores the potential of such composite nanoparticles in advancing novel drug delivery systems.
Stimuli-responsive polymers exhibit alterations in their behavior triggered by external factors such as pH, temperature, or electric fields. Poly(N-isopropylacrylamide) (PNIPAAm) homopolymer and its copolymer exemplify thermosensitive polymers. These polymers possess a distinct property: their aqueous solutions manifest a lower critical solution temperature (LCST) compared to analogous substances. Essentially, below their critical temperature, these polymers expand, effectively containing the drug or solution within. Upon surpassing the critical temperature, the polymer solution undergoes deflation, causing the contained solution or drug to be released.
Proximity to the LCST prompts even minor temperature fluctuations to induce reversible alterations in the volume and configuration of these polymers. At the LCST, an abrupt phase shift occurs, transforming the structure from an extended hydrophilic state below the threshold to a compact hydrophobic state above it. By incorporating co-monomer units [20], adjustments to the PNIPAAm homopolymer’s LCST can be made to exceed the body’s normal temperature (37 °C). The reversible nature of this phase transition renders thermosensitive polymers promising candidates for the polymeric component in temperature-responsive drug release systems.
Commonly utilized as an encapsulating layer for magnetic drug carriers, the cross-linked NIPAAm copolymer (hydrogel) facilitates continuous drug release. Furthermore, it is anticipated that drugs will remain inert during transportation at physiological temperature, yet swiftly or deliberately release upon reaching targeted tumor cells at a temperature slightly above body temperature [21,22,23].
Another variant of stimuli-responsive polymers are hydrogels sensitive to pH alterations [24]. Much like thermosensitive polymers, these hydrogels exhibit the ability to change volume in response to even minor shifts in environmental pH. In the creation of pH-dependent phase transition gels, polymers incorporating weakly acidic groups (such as acrylic acids) have conventionally been employed. Under low pH conditions, polyacid gels tend to be less swollen due to protonation of the acidic groups, rendering them non-ionized [25].
PNIPAAm possesses a unique attribute in its capacity for co-polymerization regardless of temperature fluctuations. This combination of temperature and pH sensitivity, as observed in polymers like PNIPAM and acrylic acid, equips them with the ability to encapsulate anti-cancer medications like DOX. This controlled drug delivery system safeguards encapsulated nano-carriers within the body while ensuring timely and targeted drug release within the designated cancerous tissue.
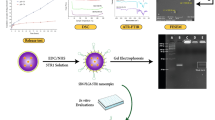
The primary aim of this study is to develop magnetic nanoparticles embedded within a responsive smart polymer for the precise delivery of drugs to targeted sites, as illustrated in Fig. 1. Our approach takes into account the considerations outlined earlier. The choice of utilizing magnetic nanoparticles was driven by their advantageous characteristics. These nanoparticles were selected for their ability to be covalently surface-modified, allowing for the attachment of Doxorubicin. The resulting conjugated system was further safeguarded through encapsulation within a synthesized smart polymer (poly(N-isopropylacrylamide-co-acrylic acid)). The various stages of this process were thoroughly assessed using spectroscopy techniques. In addition, we conducted in vitro release studies across distinct pH and temperature environments. Notably, we employed Doxorubicin, a well-established cancer therapy drug, as a representative model for our investigations.
2 Materials and Methods
2.1 Materials
The chemicals utilized in this study were procured from Sigma-Aldrich and Merck and were employed in their original form or as necessary. The list includes iron (II) sulfate (FeSO4.7H2O), manganese sulfate (MnSO4.H2O), absolute ethanol, sodium hydroxide (NaOH), methyl-3-mercaptopropionate (98.0%), methanol (99.9%), hydrazine monohydrate (98.0%), potassium persulfate (KPS), sodium dodecyl sulfate (SDS), Doxorubicin, N-isopropylacrylamide (NIPAAm, 97%), N,N-methylene bisacrylamide (BIS), and acrylic acid (AA).
2.2 Synthesis
The fabrication of the drug carrier involves a series of sequential steps. Initially, the synthesis and functionalization of metal nano-supermagnetic particles take place. Following this, the drug is linked to the functionalized particles. Finally, the resulting nanocomposite is encapsulated within a smart polymer structure responsive to changes in temperature and pH (depicted in Fig. 1).
2.3 The Synthesis of MnFe2O4 Magnetic Nanoparticles and Its Functionalization with Hydrazide End-Groups on the Surface
For the synthesis of superparamagnetic ferrite nanoparticles, the co-precipitation method was employed [26]. Initially, a solution was prepared by dissolving 7.9 g of FeSO4·7H2O and 1.8 g of MnSO4·H2O in double-distilled water under magnetic stirring. While maintaining a temperature of 14 °C, 6 mol/L NaOH was gradually introduced, and the solution was stirred for 10 min. To adjust the pH of the resulting precipitate to 7, successive washing with distilled water were performed. Ultrasound waves were applied after each wash to separate the ion particles from the precipitate. Subsequently, deoxygenation was carried out at 70 °C using nitrogen gas and magnetic stirring for a duration of 90 min. The resulting precipitate was then dried at 50 °C for 24 h.
To enhance the surface properties of MnFe2O4 magnetite nanoparticles for improved chemical bonding with the drug [15], a process called functionalization was employed. A colloidal solution was created by dispersing 150 mg of the nanoparticles in 20 mL of diphenyl ether through 3 min of ultrasound treatment. Following this, 33 µL of methyl 3-mercaptopropionate was introduced into the mixture, which was then refluxed at 200 °C for 1 h. Upon cooling to 100 °C, the solution was reflux for a 30-min reflux after the optimal addition of 146 µL of hydrazine monohydrate (Sect. 2.4). Centrifugation at 15,000 rpm for 10 min was employed to separate the nanoparticles, which were subsequently washed thrice with methanol and dried at 50 °C for 24 h.
2.4 Conjugation of Functionalized MnFe2O4 with the Drug (Drug Loading)
To prepare a colloidal solution, 150 mg of NHNH2-functionalized nanoparticles were dissolved in 20 mL of methanol. To this mixture, 150 µL of doxorubicin (110–4 mol/L) and 3 drops of acetic acid were introduced as a catalyst. After 5 min interval, ultrasound was employed to finalize the procedure. The resulting colloidal solution was then stirred for 48 h to ensure the completion of the reaction. Subsequently, centrifugation at 15,000 rpm for 10 min was performed to separate the precipitate. The drug-laden precipitate obtained through this process was subsequently subjected to a 24-h drying period at 50 °C. To determine the concentration of the unloaded drug, the Beer–Lambert law was employed, involving the recording of UV–Vis spectra of the supernatant at a wavelength of 491 nm utilizing a spectrophotometer [27]. The subsequent equation was employed to ascertain the efficiency of drug loading:
2.5 Optimization of Cross-Linking Agent
In this experiment, 1 mg of ferrite magnetic nanoparticles was functionalized with 50, 100, and 146 µL of cross-linking hydrazine monohydrate. The drug loading process involved the utilization of 304 L of Doxorubicin solution (cross-linking agent concentration = 4.8103 mol/L) [24].
Ultimately, four distinct solutions were formulated, varying in the quantity of the cross-linking agent while maintaining a consistent dosage of the drug and nanoparticles.
The first solution was prepared by adding 304 µL of the drug and 5 mL of phosphate buffer, with the drug and cross-linking agent being present at equal concentrations. The second solution was prepared by adding a mixture of 1 mg functionalized nanoparticles loaded with 304 µL drug and 146 µL cross-linking agent in 5 mL of phosphate buffer.
Solution 3: A combination of 1 mg functionalized nanoparticles loaded with 304 µL drug and 100 µL cross-linking agent in 5 mL of phosphate buffer.
Solution 4: 5 mL of phosphate buffer containing 1 mg functionalized nanoparticles loaded with 304 µL drug and 50 µL cross-linking agent.
Through the utilization of UV–Visible spectra and the application of the Beer–Lambert law, we successfully derived the concentration of unloaded Doxorubicin, which in turn facilitated the extrapolation of the loaded drug concentration. Notably, our findings revealed a proportional relationship between the quantity of cross-linking agent and the extent of drug bonding, resulting in a corresponding reduction in the availability of free Doxorubicin. In simpler terms, enhancing the number of drug receptors led to an accelerated rate of drug bonding.
2.6 The Synthesis of Smart Polymer and Encapsulation of Drug-Loaded Magnetic Nanoparticle with Smart Polymer
After synthesizing the intelligent polymer, poly(N-isopropylacrylamide-co-acrylic acid) (PNIPAAm-co-AA), we proceeded with the encapsulation of drug-loaded magnetic nanoparticles [28]. To initiate this process, a solution was prepared by dissolving 0.81 mol of isopropylacrylamide, 0.09 mol of acrylic acid, and 0.1 mol of methylenebisacrylamide in 100 mL of distilled water containing 0.15 mol of sodium dodecyl sulfate. This mixture was then subjected to agitation under a nitrogen atmosphere at 70 °C for 1 h. Subsequently, an appropriate quantity of potassium persulfate was introduced into the solution, which was then stirred for 9 h at 70 °C within a nitrogen environment. The resulting precipitate was separated through centrifugation at 15,000 rpm for 15 m and allowed to dry at room temperature over the course of 3 days.
For the subsequent step, the encapsulation of the drug-loaded magnetic nanoparticles was executed as follows: In 100 mL of distilled water, 0.81 mol of isopropylacrylamide and 0.1 mol of methylenebisacrylamide were dissolved and stirred for 30 min. To this solution, 1 mg of the drug-loaded functionalized nanoparticle was added. Meanwhile, 0.09 mol of acrylic acid and 0.15 mmol of sodium dodecyl sulfate were dissolved in 2 mL of distilled water and subsequently incorporated into the solution derived from the previous stage. The mixture was stirred at 70 °C for 1 h. Following this, the optimal amount of potassium persulfate was introduced, and the solution was stirred for 9 h, all carried out under a nitrogen atmosphere.
Ultimately, the solution was centrifuged at 15,000 rpm for 15 min to isolate the precipitate, which was then left to air-dry at room temperature for a period of 3 days.
2.7 Characterization of the Nano-carrier System
Scanning electron microscopy (SEM) (TESCAN mira3 instrument from Czechia) was used to investigate the morphology of both the synthetic polymer and the magnetic ferrite nanoparticles. Additionally, a transmission electron microscope (TEM), specifically a Philips CM30 from the Netherlands, was employed to scrutinize the structure of drug-loaded nanoparticles coated with the polymer.
Furthermore, at each stage of the process, Fourier transform infrared spectroscopy (FTIR) (PerkinElmer, USA) was used to assess the functional groups present in the synthesized compounds.
The confirmation of drug loading within the polymer PNPAAm-co-AA was achieved through the application of thermogravimetric analysis (TGA) method. A quantity of 13.246 mg of the polymer, which included drug-loaded nanoparticles, underwent TGA analysis using a PL-150 instrument from Polymer Laboratories, with a consistent heating rate. The resulting graph displaying weight variations versus temperature was subsequently scrutinized.
To further assess the nanoparticles, measurements were conducted for surface charge and particle size distribution using a zetasizer nano ZS instrument from Malvern Instruments in the UK. In drug delivery systems, particle size distribution, stability, and encapsulation efficiency stand as pivotal parameters. Zeta potential, representing the surface charge, exerts significant influence over encapsulation efficiency, colloidal stability, and the interaction of nanoparticles with their surroundings. In the context of this study, the measurements of zeta potential and particle size distribution facilitated the achievement of nanoparticle stability, uniformity, and a narrow size distribution.
2.8 LCST Measurement of the Smart Polymer
To initiate the experiment, 2 g of the synthesized polymer was introduced into 2 mL of phosphate buffer. UV–Vis absorption spectra were then systematically recorded at temperatures of 31, 33, 34, 37, 40, and 43 °C, focusing on a wavelength of 500 nm. This observation process spanned over a duration of 30 min. However, the presence of turbidity hindered the measurement of the LCST (lower critical solution temperature) within the colloidal solution containing magnetic nanoparticles coated with the smart polymer using a UV–Vis spectrophotometer. In lieu of this, the LCST was determined and subsequently graphed, with the obtained results portrayed as transmittance plotted against temperature [29].
2.9 The Study of Polymer Swelling
The investigation into polymer swelling was conducted within a range of pH levels using phosphate buffer. Initially, 0.01 g of dried polymer was distributed into vials containing distilled water adjusted to pH values of 5, 6, 7.4, and 8. An equivalent quantity of polymer was also placed into vials filled with phosphate buffer having corresponding pH levels. Following a 24-h interval, the swollen polymer was isolated and weighed utilizing filter paper. Subsequently, the swelling rate was computed through the application of the subsequent equation, in which "W" and "W0" symbolize the weights of the swollen and desiccated polymers, respectively.
In the subsequent phase of the experimentation, the rate of polymer swelling was evaluated through the utilization of Eq. (2). Initially, 0.01 g of desiccated polymer was introduced into vials containing phosphate buffer set at a consistent pH level. However, the temperatures were systematically altered to 25, 30, 35, 37, and 40 °C for a 24-h duration. Following this interval, the polymer was isolated through the application of filter paper. Subsequently, the same quantity of polymer (0.01 g) was positioned within vials that held solutions of NaCl with differing concentrations—namely, 0.001, 0.005, 0.01, 0.05, 0.1, and 0.5 M. After 24 h, filter paper was once again employed to extract the polymer, and the calculation of the polymer swelling rate was carried out using Eq. (2).
2.10 In vitro Release of Doxorubicin
The drug release profile was examined under various conditions, encompassing constant pH, differing temperatures ranging from 25 to 40 °C, and consistent temperature with variable pH values from 5 to 8. The approach included the following steps:
Under continuous stirring, 1 mg of the drug-loaded polymer was dispersed within 20 mL of phosphate buffer at pH 7.4, maintaining a temperature range spanning from 25 to 40 °C.
A 2-h interval was established for the extraction of 2 mL of the solution, which was subsequently subjected to centrifugation at 13,000 rpm for 10 min.
The resulting supernatant was separated and utilized for the study of drug release via UV–Vis spectrophotometer and Potentiostat/Galvanostat. Through a comparative analysis of the outcomes, the optimal temperature was determined. Subsequently, the investigation of drug release was carried out at the identified optimal temperature, but with varying pH values [30]. For the electrochemical determination of the drug release rate, the redox peak of doxorubicin was tracked. In essence, the study encompassed different experimental conditions to comprehensively understand the drug-releasing behavior of the system.
2.11 Cytotoxicity Study
Through the utilization of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, the in vitro cytotoxicity of doxorubicin-loaded nanoparticles was examined [31]. Hela cells were seeded in a 96-well plate, each well containing 1 mL of Dulbecco’s Modified Eagle Medium (DMEM). After an initial 24-h incubation at 37 °C and 5% CO2, a solution of N,N-dimethylethylenediamine (DMED) containing the loaded and encapsulated drug nanoparticles was introduced to each well. Subsequently, cells were incubated for 4 h at 25 °C. Further, 600 µL of fresh DMEM medium was added, and the cells were subjected to an additional 48-h incubation at 37 °C.
In the concluding phase of the procedure, 100 µL of MTT medium was dispensed into each well. Following a 4-h incubation, the MTT solution was substituted with 500 µL of Dimethyl Sulfoxide (DMSO), and agitation was carried out at room temperature. To gauge the optical density at a wavelength of 570 nm, a Microplate Reader of Model 550 was employed. Eq. (3) was utilized to derive cell viability, with Acontrol and Atreated representing the optical density values obtained in the absence and presence of doxorubicin-loaded nanoparticles, respectively.
3 Results and Discussion
3.1 Analysis Using Scanning Electron Microscopy (SEM)
Figure 2a presents a scanning electron micrograph (SEM) showcasing superparamagnetic ferrite nanoparticles with diameters ranging from 5 to 15 nm. In Fig. 2b, the synthesized polymer is depicted, revealing cavities formed under optimal conditions. These cavities emerged due to the steam evaporation during the polymerization reaction. The augmentation of cavities within the polymer’s structure leads to an enhanced speed and efficiency of absorption [32].
Given the SEM’s limitations in capturing a clearer image of the drug-loaded nanoparticles coated with the smart polymer due to their embedded state, the transmission electron microscopy (TEM) technique was employed. Figure 2c illustrates the successful encapsulation of drug-containing nanoparticles within the polymer matrix. In this representation, the dark regions correspond to magnetic ferrite nanoparticles laden with drugs, while the lighter regions denote the polymer material [33].
3.2 Characterization of Modification and Encapsulation Stages with FTIR Spectroscopy
To extend the nanoparticles’ half-life, it becomes imperative to enhance their surface quality and their ability to evade elimination by the immune system. This enhancement not only offers the advantage of improved surface interactions for drug binding but also facilitates targeted drug transport to specific sites.
Figure 3 illustrates the FTIR spectra of distinct entities: magnetic ferrite nanoparticles (a), magnetic ferrite nanoparticles functionalized with a mercapto group (b), functionalized nanoparticles loaded with doxorubicin (c), functionalized nanoparticles loaded with doxorubicin and encapsulated within the smart polymer (d), and the smart polymer itself (e).
FTIR spectra of magnetic ferrite nanoparticles (a), magnetic ferrite nanoparticles improved with the mercapto group (b), functionalized nanoparticles loaded with doxorubicin (c), functionalized nanoparticles loaded with doxorubicin that is encapsulated into the smart polymer (d), and the smart polymer (e)
The enhancement of the magnetic ferrite nanoparticles’ surface with methyl 3-mercaptopropionate results in the formation of a covalent bond between the S–H group and Fe. Subsequent functionalization with hydrazine monohydrate leads to the conversion of OCH3 to NHNH2, with the presence of an NH3+ functional group at around 1401 cm−1 confirming the successful functionalization of the magnetic ferrite nanoparticles (Fig. 3b).
Upon loading the doxorubicin drug onto the improved and functionalized ferrite manganese nanoparticles, the carbonyl group of doxorubicin reacts with the NHNH2 functional group on the nanoparticles’ surface. This interaction is evident in Fig. 3c, where the different functional groups of the ferrite manganese nanoparticles-doxorubicin conjugate are displayed. These shifts indicate the successful drug loading onto the surface of the enhanced and functionalized ferrite manganese nanoparticles.
During the encapsulation process, the drug-loaded ferrite manganese nanoparticles are enveloped with the PNIPAAm-co-AA smart polymer. The FTIR spectra (Fig. 3d) further validate the successful encapsulation of nanoparticles within the smart polymer [29, 34]. The functional groups specific to the smart polymers are exhibited in Fig. 3e.
In essence, the carbonyl group of doxorubicin reacts with the NHNH2 functional group on the enhanced and functionalized ferrite manganese nanoparticles during the drug loading phase. The distinct functional groups of the ferrite manganese nanoparticles-doxorubicin conjugate are evident in Fig. 3c. These shifts affirm the achievement of successful drug loading onto the surface of the modified ferrite manganese nanoparticles. Subsequently, the drug-loaded ferrite manganese nanoparticles are subjected to encapsulation within a layer of the PNIPAAm-co-AA smart polymer. The FTIR spectra (Fig. 3d) [29, 35] provide compelling evidence of the nanoparticle encapsulation within the smart polymer. Finally, the functional groups attributed to the smart polymers are presented in Fig. 3e.
3.3 Thermogravimetric Analysis
To substantiate the drug loading within the nanoparticles, thermogravimetric analysis (TGA) is employed to monitor the solid sample’s thermal decomposition process [36]. Figure 4 portrays the thermal decomposition behavior of both the loaded polymer and the polymer in its solitary form. When subjected to thermal processing, the loaded polymer experiences a reduction of 11.9 mg in weight, leaving only 1.1 mg of the original polymer mass remaining in solid form at 600 °C. This weight loss, indicative of mass loss, unfolds through a multi-step progression initiated by the evaporation of the polymer’s internal solvent. This is followed by the polymer’s degradation and the subsequent release of the loaded drug. The residual mass comprises metal nanoparticles that remain unaffected by the prevailing temperature. These findings affirm the successful loading of nanoparticles and showcase the polymer’s stability at temperatures below 200 °C, where only minor weight alterations occur due to solvent evaporation.
Following the evaporation of the solvent from the polymer structure (below 200 °C), a stage of stability ensues. However, at around 250 °C, the polymer’s structure begins to deteriorate and undergo decomposition. While this stage continues until the complete disintegration and decomposition of the polymer in the case of the polymer alone, the presence of the MOF structural metal within the loaded polymer causes this stage to slow down after reaching 440 °C. Consequently, a portion of the loaded polymer—comprising the structural metal—persists. The heightened thermal resistance observed in the loaded polymer can be attributed to the influence of the MOF on the polymer’s decomposition, resulting in an augmentation of its thermal resilience.
3.4 Potential Measurement
The surface characteristics of nanoparticles play a pivotal role in targeted drug delivery. In the bloodstream, unmodified nanoparticles can swiftly be eliminated by fixed macrophages of the reticuloendothelial system (RES). To counteract this phagocytosis process and enable nanoparticles to remain in circulation for an extended duration, surface modification stands as one of the most prevalent strategies. Alterations in the surface electric charge of nanoparticles transpire during their modification, drug conjugation, and polymer encapsulation.
The Zeta (ζ) potential serves as an electrokinetic parameter that directly correlates with the actual surface charge. By measuring the ζ-potential, one can determine the concentration, distribution, adsorption, ionization, exposure, or shielding of charged moieties. This measurement stands as the simplest and most fundamental method to characterize the surface charge of colloids, with its unit being the millivolt [37].
While Zeta potential cannot be directly measured, it can be derived through electrophoretic movement. A drop in electric potential below the critical point can disturb the charged double layers surrounding particles, leading to their aggregation. A Zeta potential of ± 25 mV serves as a threshold to differentiate the strength of electrical charges on the charged layers’ surfaces. In scenarios devoid of hindrances like high viscosity and spatial constraints, particles with low Zeta potential (positive or negative charge) are more prone to coagulation.
The presence of carbonyl functional groups within the polymer’s structure contributes to a negative surface potential (− 18.2) for the polymeric nanocapsules (Fig. 5). Similarly, the entry of drug-loaded magnetic nanoparticles into the polymeric capsule induces a minor reduction in surface charge to − 20.8, attributable to the carbonyl functional group present in the structure of doxorubicin. This change can be explained by the similarity in charges between the polymer and the drug, as evidenced by their functional groups. Collectively, these observations provide compelling evidence of a successful drug loading process.
3.5 Measuring the Polydispersity Index of the Nanoparticles in the Solvent
For the assessment of nanoparticle size distribution within a solvent, scientists employ a parameter known as the polydispersity index (PDI) [38]. A PDI value approaching 0.7 signifies a narrow size distribution and uniformity among the nanoparticles, while a PDI near 1.0 suggests a broader size distribution within the solvent. The calculation of PDI is accomplished using the equation provided as follows:
where d denotes the size of the nanoparticles and σ denotes the standard deviation. As it can be observed in Fig. 6, the calculated amount of PDI for nanocapsules and loaded nanocapsules are approximately near 0.7, implying the acceptable distribution dispersion of nanocapsules before and after the drug loading.
3.6 The LCST Determination of the PNIPAAm-co-AA
As depicted in Fig. 7, the polymer exhibits a single-phase nature within the temperature range of 31–37 °C. During this interval, the prevailing dominance of hydrogen bonds between the polymer chains and water molecules imparts a hydrophilic character to the polymer. However, upon reaching temperatures spanning 39–43 °C, the polymer undergoes a distinctive two-phase transition. This transition unveils the intramolecular bonds between the C–O and N–H groups, contributing to the formation of the hydrophobic polymer state. Notably, the critical temperature is positioned at 38.5 °C, approximately at the midpoint of the temperature range encompassing the phase change.
At this critical temperature, even minor fluctuations in temperature provoke reversible alterations in the polymer’s shape and volume. As demonstrated in Fig. 7, the enthalpy associated with hydrogen bonds involving water molecules is surpassed by the overall increase in entropy. Consequently, the LCST (Lower Critical Solution Temperature) corresponds to the region on the graph where this phenomenon occurs. To a significant extent, this phenomenon hinges upon the interplay of hydrogen bonds among the polymer’s constituent monomers [39].
3.7 Effect of Temperature, pH, and Ionic Strength on Polymer Swelling
An exploration into the impact of pH on polymer swelling was investigated. In a phosphate buffer solution, the highest degree of swelling was observed at a pH value of 8 (Fig. 8a). This phenomenon is attributed to an augmented absorption of water due to the elimination of the possibility of hydrogen bonding, consequently resulting in an amplified level of swelling.
In contrast, at pH 5, the stability of the acrylamide/acrylic acid complex is heightened as a consequence of the transformation of COONH2 to COONH3+. This transformation leads to intensified electrostatic binding between the polymer and the acid, subsequently leading to a reduction in swelling. Enhanced electrostatic and hydrogen bonds between acrylamide and acrylic acid at pH 6 and 7 contributed to a decrease in swelling [40]. Figure 8b presents an analysis of the swelling rate across a spectrum of temperatures, encompassing 25, 30, 35, 37, and 40 °C. It was discerned that higher temperatures, such as 40 °C, correlate with reduced swelling. Conversely, lower temperatures exhibit an elevation in swelling. The higher temperatures prompt the formation of hydrogen bonds and other intramolecular interactions among polymer molecules, culminating in a more compact and robust material structure. This compactness hinders the entrance of water molecules, ultimately leading to diminished swelling.
The outcomes of polymer swelling under varied ionic strengths are delineated in Fig. 8c. These findings underscore that heightened ionic strength results in diminished polymer swelling. This is attributed to a decline in osmotic pressure between the polymeric phase and the aqueous phase, causing the polymer to contract to the extent that water absorption becomes impeded. This phenomenon is underpinned by an increase in both solution ionic strength and the polymer’s electrical charge. Notably, it was ascertained that 0.001 M NaCl yielded the most substantial swelling, while 0.1 M NaCl yielded the least swelling [41].
3.8 Analysis of Drug Loading Efficiency into Poly-PNIPAAm-co-AA Containing Functionalized Magnetic Nanoparticle
While the initial drug loading efficiency for functionalized nanoparticles was determined to be 60.77 using Eq. (1), this value underwent modification within the polymeric matrix containing enhanced nanoparticles due to the amplified polymer swelling in the presence of a solvent. The acceleration of drug loading within the polymeric framework can be achieved by elevating the swelling rate. Over distinct time intervals of 2, 4, 6, 8, and 24 h, the drug loading efficiency manifested as 8.43%, 17.63%, 27.61%, 43.13%, and 71.52%, respectively. Upon a comprehensive data analysis, a 24 h duration emerged as the optimal timeframe for efficient drug loading. The extent of saturation during the introduction of the polymeric solution significantly impacts the quantity of successfully loaded drug. With the passage of time, the loading rate experiences an upward trajectory before eventually stabilizing. This dynamic can be attributed to the polymer’s inherent intelligence, characterized by interconnected chains bound via cross-linking bonds. Moreover, the polymer is endowed with ionic properties or functional groups, and the polymer’s cavities exhibit a notable affinity for water absorption [42]. Consequently, the polymer’s propensity to absorb water persists, resulting in progressive expansion over time.
3.9 Drug In vitro Releasing Behavior
Prior to conducting in vivo drug testing, it is imperative to assess its in vitro diffusion behavior. Nonetheless, by utilizing phosphate buffer at pH 7.4 and a temperature of 37 °C, a more plausible emulation of human physiological conditions is feasible. This approach, although unable to replicate the intricacies of the human body’s circulatory, digestive, urinary, and nervous systems precisely, provides a close approximation. The evaluation of doxorubicin release was conducted under diverse conditions through an electrochemical method. The polymer exhibits structural modifications under varying conditions, including pore opening and closure, which intricately influence the polymer–solvent interaction, subsequently impacting the drug’s entrance and exit rate.
The release of the drug is profoundly contingent on temperature, owing to the inherent thermosensitivity of the PNIPAAm-co-AA polymer. This temperature dependency is attributed to alterations in pore diameter resulting from polymer structural transitions at different temperatures, intricately affecting drug release dynamics. As depicted in Fig. 9a, drug release profiles were scrutinized at 25, 30, 35, 37, and 40 °C. Notably, the absence of discernible doxorubicin peaks at 25, 30, or 35 °C implies minimal drug release at these temperatures. This phenomenon is attributable to the polymer’s hydrophilic nature below the critical temperature, facilitating aqueous solution absorption and polymer expansion, subsequently sealing the nanocapsule pores and impeding drug release.
The peaks manifested at 37 °C indicate a relatively modest drug release at this temperature. This observation is rooted in the proximity of this temperature to the critical temperature, signifying a phase transition of the polymer wherein drug release is limited. Evidently, the emergence of doxorubicin peaks at 40 °C, surpassing the critical temperature, suggests facilitated drug release due to open pores within the polymer structure.
Notably, cancerous tissues typically exhibit temperatures exceeding 40 °C, surpassing those of normal tissues, thereby augmenting drug release. The marginal drug release observed at 25 °C serves as a rationale for maintaining the drug at this temperature [43, 44].
Environmental factors such as pH profoundly impact the formation of acidic and alkaline groups within polymer structures, influencing their hydrogen-binding capabilities. Augmented interactions within the polymer structure engender densification, leading to expanded pores. Enhanced porosity, ensuing from heightened interactions, can govern molecular ingress and egress rates. Variations in pH modulate the hydrogen-binding propensity of involved functional groups, thereby influencing drug release behavior. Consequently, the release of the drug was evaluated across different time frames within acidic, neutral, and alkaline environments.
Given the pH sensitivity of acrylic acid, an element of the synthesized copolymer, the polymer demonstrates minimal swelling at acidic pH levels, thus promoting optimal doxorubicin release, as illustrated in Fig. 9b. The polymeric capsules exhibit the capacity for controlled drug release, enhancing therapeutic efficacy, a facet further accentuated by the fact that cancerous tissues typically harbor pH levels of 5–5.3 [45]. This strategy curtails drug-related side effects within healthy tissues, characterized by pH levels approximating neutrality at 7.4. To bolster the stability of drug-loaded nanoparticles, storing them within an alkaline pH milieu could effectively minimize drug release.
3.10 The Antitumor Effect of DOX-Loaded Poly NIPAAm-co-AA/MnFe2O4 System
Cytotoxicity assessment was employed to evaluate the antitumor effectiveness of the polymeric nano-carrier. Cells were co-incubated with drug-loaded magnetic nanoparticles and free DOX nano-carriers at 25 °C for 4 h as a baseline control. The initiation of drug release occurred through a temperature increase to 40 °C. As depicted in Fig. 10, the antitumor impact of DOX and DOX-loaded nanoparticles shows minimal effect at low drug concentrations, but experiences a substantial escalation at higher drug concentrations.
At a drug concentration of 50 µg/L, the viability of cultured cells co-incubated with magnetic nanoparticles-DOX, DOX-loaded smart polymer, and free DOX stands at 99%, 59%, and 27%, respectively. These findings underline the heightened cytotoxicity of DOX-loaded smart polymer compared to free DOX and magnetic nanoparticles.
According to several studies, nanoparticles can be internalized into cells via the endocytosis pathway and subsequently localize within endosomes. Encapsulating the anti-cancer drug within nanoparticles and a smart polymer can enhance the drug’s entry into cells and its retention within lysosomes. In our current investigation, the augmented cellular uptake of drug-loaded nanoparticles might lead to amplified cytotoxicity due to the specific attributes of the drug-loaded nanoparticles that facilitate drug penetration, along with the contributions of nano-carrier-mediated processes [46].
4 Conclusions
This study has successfully designed a magnetic drug delivery system that displays sensitivity to variations in temperature and pH. In contrast to similar endeavors, this research has implemented a straightforward and precise method to construct a responsive nanocarrier. The assemblage comprises MnFe2O4 magnetic nanoparticles that have undergone functionalization with hydrazide end-groups, subsequently being loaded with doxorubicin. These nanoparticles were then enshrouded within a poly(NIPAAm-co-AA) smart polymer possessing a lower critical solution temperature (LCST) of 38.5 °C. In vitro investigations indicated that drug release was influenced by both temperature and pH, with heightened releases occurring at temperatures exceeding the LCST (40 °C) and within a pH range of 5–5.3.
Furthermore, the DOX-loaded magnetic smart polymer showcased an amplified cytotoxic effect in comparison to the efficacy of the free drug. These outcomes propose that the magnetic drug delivery system established in this study holds potential as a promising novel approach for cancer treatment. Further research is imperative to fine-tune the system and to evaluate its effectiveness in an in vivo context.
References
Gholivand, M.B.; Peyman, H.; Gholivand, K.; Roshanfekr, H.; Taherpour, A.A.; Yaghobi, R.: Theoretical and instrumental studies of the competitive interaction between aromatic α-aminobisphosphonates with DNA using binding probes. Appl. Biochem. Biotechnol. 182(3), 925–943 (2017)
Kashanian, S.; Khodaei, M.M.; Roshanfekr, H.; Peyman, H.: DNA interaction of [Cu (dmp)(phen-dion)](dmp= 4, 7 and 2, 9 dimethyl phenanthroline, phen-dion= 1, 10-phenanthroline-5, 6-dion) complexes and DNA-based electrochemical biosensor using chitosan–carbon nanotubes composite film. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 114, 642–649 (2013)
Abdollahy, M.; Peyman, H.; Roshanfekr, H.; Idris, A.O.; Azizi, S.; Sibali, L.L.: Synthesis and characterization of a smart polymer-coated core–shell MnFe2O4@ organometallic framework for targeted drug delivery. Chem. Pap. 77(7), 3897–3909 (2023)
Akhlaghi, N.; Najafpour-Darzi, G.: Manganese ferrite (MnFe2O4) nanoparticles: from synthesis to application—a review. J. Ind. Eng. Chem. 103, 292–304 (2021)
Ceylan, M.; Misak, H.E.; Strong, N.; Yang, S.Y.; Asmatulu, R.: Reduced toxicity of protein/magnetic targeted drug delivery system for improved skin cancer treatment in mice model. J. Magn. Magn. Mater. 539, 168404 (2021)
Salih, S.J.; Mahmood, W.M.: Review on magnetic spinel ferrite (MFe2O4) nanoparticles: from synthesis to application. Heliyon 9(6), e16601 (2023)
Tokuyama, H.; Mori, H.; Hamaguchi, R.; Kato, G.: Prediction of the lower critical solution temperature of poly(N-isopropylacrylamide-co-methoxy triethyleneglycol acrylate) in aqueous salt solutions using support vector regression. Chem. Eng. Sci. 231, 116325 (2021)
Nagy, Ľ; Zeleňáková, A.; Hrubovčák, P.; Barutiak, M.; Lisnichuk, M.; Bednarčík, J., et al.: The role of pH on the preparation of citric acid coated cobalt ferrite nanoparticles for biomedical applications. J. Alloys Compd. 960, 170833 (2023)
Bellotti, E.; Schilling, A.L.; Little, S.R.; Decuzzi, P.: Injectable thermoresponsive hydrogels as drug delivery system for the treatment of central nervous system disorders: a review. J. Control. Release 329, 16–35 (2021)
Kalaiselvan, C.R.; Laha, S.S.; Somvanshi, S.B.; Tabish, T.A.; Thorat, N.D.; Sahu, N.K.: Manganese ferrite (MnFe2O4) nanostructures for cancer theranostics. Coord. Chem. Rev. 473, 214809 (2022)
Fan, Y.; Cui, Y.; Hao, W.; Chen, M.; Liu, Q.; Wang, Y., et al.: Carrier-free highly drug-loaded biomimetic nanosuspensions encapsulated by cancer cell membrane based on homology and active targeting for the treatment of glioma. Bioact. Mater. 6(12), 4402–4414 (2021)
Roslan, A.A.; Aziz, N.A.A.; Deraman, N.F.A.; Dzulkarnain, I.: Effects of synthesis parameters on the performance of crosslinked co-polymers with clays for conformance control. Pet. Res. (2023). https://doi.org/10.1016/j.ptlrs.2023.07.006
Turrina, C.; Schoenen, M.; Milani, D.; Klassen, A.; Rojas Gonzaléz, D.M.; Cvirn, G., et al.: Application of magnetic iron oxide nanoparticles: thrombotic activity, imaging and cytocompatibility of silica-coated and carboxymethyl dextrane-coated particles. Colloids Surf. B Biointerfaces 228, 113428 (2023)
Liu, X.; Liu, Y.; Guo, Y.; Shi, W.; Sun, Y.; He, Z., et al.: Metabolizable pH/H2O2 dual-responsive conductive polymer nanoparticles for safe and precise chemo-photothermal therapy. Biomaterials 277, 121115 (2021)
Gambhir, R.P.; Rohiwal, S.S.; Tiwari, A.P.: Multifunctional surface functionalized magnetic iron oxide nanoparticles for biomedical applications: a review. Appl. Surf. Sci. Adv. 11(May), 100303 (2022)
Shah, S.A.; Majeed, A.; Rashid, K.; Awan, S.U.: PEG-coated folic acid-modified superparamagnetic MnFe2O4 nanoparticles for hyperthermia therapy and drug delivery. Mater. Chem. Phys. 138(2–3), 703–708 (2013)
Malakootikhah, J.; Rezayan, A.H.; Negahdari, B.; Nasseri, S.; Rastegar, H.: Porous MnFe2O4@SiO2 magnetic glycopolymer: a multivalent nanostructure for efficient removal of bacteria from aqueous solution. Ecotoxicol. Environ. Saf. 166, 277–284 (2018)
Guisasola, E.; Vallet-Regí, M.; Baeza, A.: Magnetically responsive polymers for drug delivery applications. In: Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications: Volume 1: Types and Triggers, pp. 143–168. Elsevier (2018)
Gholipour, N.; Akhlaghi, M.; Mokhtari Kheirabadi, A.; Geramifar, P.; Beiki, D.: Development of Ga-68 labeled, biotinylated thiosemicarbazone dextran-coated iron oxide nanoparticles as multimodal PET/MRI probe. Int. J. Biol. Macromol. 148, 932–941 (2020)
Conzatti, G.; Ayadi, F.; Cavalie, S.; Carrère, N.; Tourrette, A.: Thermosensitive PNIPAM grafted alginate/chitosan PEC. Appl. Surf. Sci. 467, 940–948 (2019)
Alsehli, M.: Polymeric nanocarriers as stimuli-responsive systems for targeted tumor (cancer) therapy: recent advances in drug delivery. Saudi Pharm. J. 28(3), 255–265 (2020)
Dhamecha, D.; Le, D.; Chakravarty, T.; Perera, K.; Dutta, A.; Menon, J.U.: Fabrication of PNIPAm-based thermoresponsive hydrogel microwell arrays for tumor spheroid formation. Mater. Sci. Eng. C 125(April), 112100 (2021)
Carreño, G.; Pereira, A.; Ávila-Salas, F.; Marican, A.; Andrade, F.; Roca-Melendres, M.M., et al.: Development of “on-demand” thermo-responsive hydrogels for anti-cancer drugs sustained release: rational design, in silico prediction and in vitro validation in colon cancer models. Mater. Sci. Eng. C Mater. Biol. Appl. 131(July), 112483 (2021)
Omer, A.M.; Sadik, W.A.A.; El-Demerdash, A.G.M.; Hassan, H.S.: Formulation of pH-sensitive aminated chitosan–gelatin crosslinked hydrogel for oral drug delivery. J. Saudi Chem. Soc. 25(12), 101384 (2021)
Kubo, M.; Higuchi, M.; Koshimura, T.; Shoji, E.; Tsukada, T.: Control of the temperature responsiveness of poly(N-isopropylacrylamide-co-2-hydroxyethyl methacrylate) copolymer using ultrasonic irradiation. Ultrason. Sonochem. 79, 105752 (2021)
Akhtar, M.J.; Younas, M.: Structural and transport properties of nanocrystalline MnFe2O4 synthesized by co-precipitation method. Solid State Sci. 14(10), 1536–1542 (2012)
Araújo, F.; das Neves, J.; Martins, J.P.; Granja, P.L.; Santos, H.A.; Sarmento, B.: Functionalized materials for multistage platforms in the oral delivery of biopharmaceuticals. Prog. Mater. Sci. 89, 306–344 (2017)
Fan, T.; Li, M.; Wu, X.; Li, M.; Wu, Y.: Preparation of thermoresponsive and pH-sensitivity polymer magnetic hydrogel nanospheres as anticancer drug carriers. Colloids Surf. B Biointerfaces 88(2), 593–600 (2011)
Kamoun, E.A.; Fahmy, A.; Taha, T.H.; El-Fakharany, E.M.; Makram, M.; Soliman, H.M.A., et al.: Thermo-and pH-sensitive hydrogel membranes composed of poly(N-isopropylacrylamide)-hyaluronan for biomedical applications: influence of hyaluronan incorporation on the membrane properties. Int. J. Biol. Macromol. 106, 158–167 (2018)
Khan, I.; Sarkar, B.; Joshi, G.; Nakhate, K.T.; Ajazuddin, M.A.K., et al.: Biodegradable nanoparticulate co-delivery of flavonoid and doxorubicin: mechanistic exploration and evaluation of anticancer effect in vitro and in vivo. Biomater. Biosyst. 3, 100022 (2021)
Lee, S.Y.; Koo, I.S.; Hwang, H.J.; Lee, D.W.: In Vitro three-dimensional (3D) cell culture tools for spheroid and organoid models. SLAS Discov. 28(4), 119–137 (2023)
Elhaddad, E.; Al-fawwaz, A.T.: Synthesis, characterization and mechanism of MnFe2O4@g-C3N4 nanocomposite as an effective photocatalyst for the generation of hydrogen and organic contamination degradation. Egypt. J. Pet. 32(2), 41–46 (2023)
Ma, Y.; Xu, X.; Lu, L.; Meng, K.; Wu, Y.; Chen, J., et al.: Facile synthesis of ultrasmall MnFe2O4 nanoparticles with high saturation magnetization for magnetic resonance imaging. Ceram. Int. 47(24), 34005–34011 (2021)
Singh, G.; Chandra, S.: Electrochemical performance of MnFe2O4 nano-ferrites synthesized using thermal decomposition method. Int. J. Hydrogen Energy 43(8), 4058–4066 (2018)
Ma, J.; Sun, J.; Fan, L.; Bai, S.; Panezai, H.; Jiao, Y.: Fractal evolution of dual pH- and temperature-responsive P(NIPAM-co-AA)@BMMs with bimodal mesoporous silica core and coated-copolymer shell during drug delivery procedure via SAXS characterization. Arab. J. Chem. 13(2), 4147–4161 (2020)
Ribeiro, A.M.; Ramalho, E.; Neto, M.P.; Pilão, R.M.: Thermogravimetric analysis of high-density cork granules using isoconversional methods. Energy Rep. 8, 442–447 (2022)
Andrea, F.; Gust, R.; Bernkop-schn, A.; Maria, A.: Zeta potential shifting nanoemulsions comprising single and gemini tyrosine-based surfactants. Eur. J. Pharm. Sci. 189, 106538 (2023)
Ghahramani, N.; Rahmati, M.: The effect of the molecular weight and polydispersity index on the thermal conductivity of polyamide 6: a molecular dynamics study. Int. J. Heat Mass Transf. 154, 119487 (2020)
Metawea, O.R.M.; Abdelmoneem, M.A.; Haiba, N.S.; Khalil, H.H.; Teleb, M.; Elzoghby, A.O., et al.: A novel ‘smart’ PNIPAM-based copolymer for breast cancer targeted therapy: synthesis, and characterization of dual pH/temperature-responsive lactoferrin-targeted PNIPAM-co-AA. Colloids Surf. B Biointerfaces 202(March), 111694 (2021)
Zhan, Y.; Fu, W.; Xing, Y.; Ma, X.; Chen, C.: Advances in versatile anti-swelling polymer hydrogels. Mater. Sci. Eng. C 127(March), 112208 (2021)
Yu, Z.; Tsen, W.C.; Qu, T.; Cheng, F.; Hu, F.; Liu, H., et al.: Highly ion-conductive anion exchange membranes with superior mechanical properties based on polymeric ionic liquid filled functionalized bacterial cellulose for alkaline fuel cells. J Mater Res Technol. 23, 6187–6199 (2023)
Li, K.; Zang, X.; Cheng, M.; Chen, X.: Stimuli-responsive nanoparticles based on poly acrylic derivatives for tumor therapy. Int. J. Pharm. 601(March), 120506 (2021)
Liu, X.; Guo, Z.; Ge, T.; Hu, J.; Wang, J.; Yang, L.: Self-assembly and in vitro drug release behaviors of amphiphilic copolymers based on functionalized aliphatic liquid crystalline polycarbonate with pH/temperature dual response. J. Mol. Liq. 316, 113837 (2020)
Bratek-Skicki, A.: Towards a new class of stimuli-responsive polymer-based materials—recent advances and challenges. Appl. Surf. Sci. Adv. 4, 100068 (2021)
Zhalechin, M.; Dehaghi, S.M.; Najafi, M.; Moghimi, A.: Magnetic polymeric core-shell as a carrier for gradual release in-vitro test drug delivery. Heliyon 7(5), e06652 (2021)
Misiak, P.; Niemirowicz-Laskowska, K.; Misztalewska-Turkowicz, I.; Markiewicz, K.H.; Wielgat, P.; Car, H., et al.: Doxorubicin delivery systems with an acetylacetone-based block in cholesterol-terminated copolymers: diverse activity against estrogen-dependent and estrogen-independent breast cancer cells. Chem. Phys. Lipids 245, 105194 (2022)
Funding
Open access funding provided by University of South Africa.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is not conflict of interest for this paper.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jafarzadeh, F., Peyman, H., Roshanfekr, H. et al. Fabrication of a Nanomagnetic Smart Polymer Carrier as a Potential Candidate for a Drug Delivery System. Arab J Sci Eng 49, 9381–9394 (2024). https://doi.org/10.1007/s13369-024-08724-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-024-08724-0