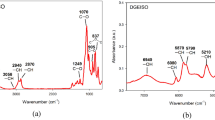
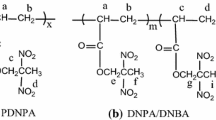
Abstract
Cure kinetics of the reaction of diglycidyl ether of bisphenol A with inorganic complexes based on zinc (II) chelate with diethylene triamine (Dien) as ligand were studied using non-isothermal differential scanning calorimetry (DSC). The complex curing agents were synthesized and characterized by elemental analysis, FT-IR, and ICP- Plasma techniques. Thermal dissociation behaviour of curing agents was also studied using thermogravimetric (TG) analysis in isolated form. The parameters of non-isothermal curing kinetics, activation energy (Ea), pre-exponential factor (A) and rate constant (K) were obtained according to Kissinger, Ozawa, and iso-conversion equations. The activation energy values for DGEBA/Zn(Dien)2Br2 and DGEBA/Zn(Dien)2(NO3)2 systems obtained by Kissinger method were 106.50 and 86.95, by Ozawa method were 108.63 and 91.12 kJ/mol and by iso-conversion equation were 107.67 and 92.66 kJ/mol, respectively. The values for pre-exponential factor (A) obtained for DGEBA/Zn(Dien)2Br2 and DGEBA/Zn(Dien)2(NO3)2 systems were 4.80 × 1011 and 4.44 × 107 s−1 with rate constants (K) of 0.90 and 0.12 s−1, respectively. The DSC thermograms of DGEBA with bromide complex showed two exothermic peaks, while DGEBA curing with nitrate complex displayed only one isolated peak.












Similar content being viewed by others
References
Peltola J, Cao Y, Smith P (1995) Epoxy adhesives made with inherently conducting polymers. Adhes Age 38(5):18–20
Hamerton I, Howlin BJ, Jepson P (2002) Metals and coordination compounds as modifiers for epoxy resins. Coord Chem Rev 224(1):67–85
Najat S, Adnan AR (2011) A study mechanical properties of epoxy resin cured at constant curing time and temperature with different hardeners. 29:1804−1818
Kurnoskin AV (1993) Metalliferous epoxy chelate polymers: 1. Synthesis and properties. Polymer 34(5):1060–1067
Kurnoskin A (1992) Reaction mechanisms of the metal chelates with epoxy oligomers and the structures of the epoxy—chelate metal‐containing matrixes. J Appl Polym Sci 46(9):1509–1530
Chen WY, Wang YZ, Chang FC (2004) Study on curing kinetics and curing mechanism of epoxy resin based on diglycidyl ether of bisphenol A and melamine phosphate. J Appl Polym Sci 92(2):892–900
Ghaemy M, Barghamadi M, Behmadi H (2004) Cure kinetics of epoxy resin and aromatic diamines. J Appl Polym Sci 94(3):1049–1056
Ghaemy M, Omrani A, Rostami A (2005) Study of reaction kinetics of epoxy and a nickel (II) complex using dynamic DSC technique. J Appl Polym Sci 97(1):265–271
Itoh T, Fujii Y, Tada T, Yoshikawa Y, Hisada H (1996) Thermodynamic and kinetic studies of zinc (II)-triamine complexes as models of CA and AP. Bull Chem Soc Jpn 69(5):1265–1274
Withersby MA, Blake AJ, Champness NR, Cooke PA, Hubberstey P, Li W-S, Schröder M (1999) Solvent control in the synthesis of 3, 6-bis (pyridin-3-yl)-1, 2, 4, 5-tetrazine-bridged cadmium (II) and zinc (II) coordination polymers. Inorg Chem 38(10):2259–2266
Curtis N (1964) 507. Some cyclic tetra-amines and their metal-ion complexes. Part I. Two isomeric hexamethyltetra-azacyclotetradecanes and their copper (II) and nickel (II) complexes. J Chem Soc:2644–2650
Naiini AA, Young V, Verkade JG (1995) New complexes of thriethanolamine (Tea): Novel structural features of [Y(TEA)2](ClO4)3 · 3C5H5N and [Cd(TEA)2](NO3)2. Polyhedron 14(3):393–400
Hague DN, Moreton AD (1987) Complexes formed between zinc(II) and diethylenetriamine: a carbon-13 nuclear magnetic resonance study. J Chem Soc Dalton Trans 12:2889–2895
Zsakó J, Pokol G, Novák C, Várhelyi C, Dobó A, Liptay G (2001) Kinetic analyis of tg data xxxv. Spectroscopic and thermal studies of some cobalt (III) chelates with ethylenediamine. J Therm Anal Calorim 64(2):843–856
Zsakó J, Várhelyi M, Várhelyi C (1979) Kinetic analysis of thermogravimetric data XIII. Thermal decomposition of complexes of type [Co(en)2(pyridine)Cl]X 2. J Therm Anal Calorim 17(1):123–131
Curtis NF, Powell HKJ (1968) Some complexes of diethylenetriamine with nickel(II), copper(II), and zinc(II). J Chem Soc A: Inorg Phys Theor (0):3069−3073
Mohammadi SR, Khonakdar HA, Ehsani M, Jafari SH, Wagenknecht U, Kretzschmar B (2011) Investigation of thermal behavior and decomposition kinetic of PET/PEN blends and their clay containing nanocomposites. J Polym Res 18(6):1765–1775
Du M, Guo B, Wan J, Zou Q, Jia D (2010) Effects of halloysite nanotubes on kinetics and activation energy of non-isothermal crystallization of polypropylene. J Polym Res 17(1):109–118
Kissinger HE (1957) Reaction kinetics in differential thermal analysis. Anal Chem 29(11):1702–1706
Nam JD, Seferis JC (1993) Application of the kinetic composite methodology to autocatalytic‐type thermoset prepreg cures. J Appl Polym Sci 50(9):1555–1564
Ozawa T (1970) Kinetic analysis of derivative curves in thermal analysis. J Therm Anal Calorim 2(3):301–324
Ramis X, Cadenato A, Morancho J, Salla J (2003) Curing of a thermosetting powder coating by means of DMTA, TMA and DSC. Polymer 44(7):2067–2079
Zhou T, Gu M, Jin Y, Wang J (2006) Isoconversional method to explore the cure reaction mechanisms and curing kinetics of DGEBA/EMI2, 4/nano SiC system. J Polym Sci A Polym Chem 44(1):371–379
Acknowledgments
The authors would like to acknowledge the financial support of the work by the Universiti Kebangsaan Malaysia and Centre of Research and Innovation Management (CRIM), UKM.
Conflict of interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghoreishi, K., Yarmo, M.A. & Asim, N. Investigation of curing kinetics of diglycidyl ether of bisphenol A and zinc (II) complexes using dynamic DSC technique. J Polym Res 22, 18 (2015). https://doi.org/10.1007/s10965-014-0653-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-014-0653-7