Abstract
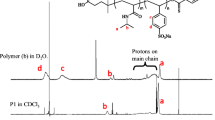
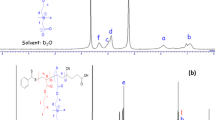
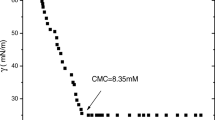
This study describes the surface, micellar, associative and thermodynamic properties of four diblock oxyethylene (E)/oxybutylene (B) copolymers with different hydrophilic block ends and various hydrophilic/hydrophobic ratios in aqueous media. The copolymers were denoted DE40B18, TE40B18, E56B19 and E56B7. The aqueous polymer solutions at various concentrations and temperatures were investigated by surface tensiometry and dynamic and static laser light scattering. Surface tension measurements were employed to detect the critical micelle concentration (CMC) as well as to calculate the surface-active and thermodynamic parameters of adsorption at the air/water interface. CMC values were also used to calculate the enthalpy of micellization (∆H 0 mic), free energy of micellization (∆G 0 mic) and entropy of micellization (∆S 0 mic). Similarly, various thermodynamic parameters for adsorption at the air/water interface were also deduced. Dynamic light scattering (DLS) was used to obtain the hydrodynamic radii (r h) and volumes (υ h) of the micelle at different temperatures, and hence the hydrodynamic expansion parameter (δ h) was also estimated. Likewise, static light-scattering measurements enabled us to determine various parameters of the copolymer micelles, such as the weight-average molar mass (M w), association number (N w), thermodynamic radius (r t), thermodynamic volume (υ t), anhydrous volume (υ a) and the thermodynamic expansion parameter (δt). Various thermodynamic and micellar parameters obtained from light scattering show that the micelles formed are spherical in shape and have rather soft interaction potentials at low temperature but become harder at higher temperature. Based on the different experimental results obtained, it can be said that various surface, micellar and thermodynamic parameters are dependent not only on the temperature and solution conditions but also on the hydrophobic/hydrophilic ratio and the end-group composition of the polymer. Modification of the hydrophilic end group of the polymer prominently affects various micellar properties. This effect can be assigned to the difference in polarity and the intermicellar charge effect.




Similar content being viewed by others
References
Roler A, Vandermeulen GWM, Klok H (2001) Adv Drug Deliv Rev 53:95–108
Talom RM, Fuks G, Mingotaud C, Gineste S, Gauffre F (2012) J Colloid Interface Sci 387:180–186
Du J, Chen Y, Zhang Y, Han CC, Fischer K, Schmidt M (2003) J Am Chem Soc 125:14710–14711
Dech S, Wruk V, Fik CP, Tiller JC (2012) Polymer 53:701–707
Antonietti M, Forster S, Hartmann J, Oestreich S (1996) Macromolecules 29:3800–3806
Mayer ABR, Mark JE (1997) Colloid Polym Sci 275:333–340
Rao J, Zhang J, Xu J, Liu S (2008) J Colloid Interf Sci 328:196–202
Hadjichristidis N, Pispas S, Floudas GA (2003) Block copolymers. Synthetic strategies, physical properties and applications. Wiley, New York
Hamley W (1998) The physics of block copolymers. Oxford University Press, Oxford
Booth C, Attwood D (2000) Macromol Rapid Commun 21:501–527
Jones MC, Gao H, Leroux C (2008) J Control Release 132:208–215
Yu K, Esenberg A (1998) Macromolecules 31:3509–3518
Cambón A, Rey-Rico A, Barbosa S, Soltero JFA, Yeates SG, Brea J, Loza MI, Alvarez-Lorenzo C, Concheiro A, Taboada P, Mosquera V (2013) J Control Release 167:68–75
Iijma M, Nagasaki Y, Okada T, Kato M, Kataoka K (1999) Macromolecules 2:1140–1146
Otsuka U, Nagasaki Y, Kataoka K, Okano T, Sakurai Y (1998) Polym Prepr 9:128–129
Spatz JP, Herzog T, Mobmer S, Ziemann P, Moller M (1999) Adv Mater 11:149–153
Liu G (2000) Chin J Polym Sci 18:255–262
Jenekhe SA, Chen XL (1999) Science 283:372–375
Yuan J, Xu Z, Cheng S, Feng L (2002) Eur Polym J 38:1537–1546
Booth C, Yu GE, Nace VM (2000) In: Alexandridis P, Lindman B (eds) Amphiphilic block copolymers: self-assembly and applications. Elsevier, Amsterdam, pp 57–86
Booth C, Attwood D, Price C (2006) Phys Chem Chem Phys 8:3612–3622
Alexandridis P (1997) Curr Opin Colloid Interface Sci 2:478–489
Mata J, Joshi T, Varade D, Ghosh G, Bahadur P (2004) Colloid Surf A 247:1–7
Castro E, Tabooda P, Mosquera V (2005) J Phys Chem B 109:5592–5599
Li X, Wettig SD, Verrall RE (2005) J Colloid Interf Sci 282:466–477
Desai H, Varade D, Aswal VK, Goyal PS, Bauhaus P (2006) Eur Polym J 42:593–601
Jain NJ, Aswal VK, Goyal PS, Bahadur P (2000) Colloid Surf A 173:85–94
Ganguly R, Aswal VK, Hassan PA, Gopalakrishnan IK, Yakhmi JV (2005) J Phys Chem B 109:5653–5658
Castro E, Tabooda P, Mosquera V (2006) J Phys Chem B 110:13113–13123
Khan A, Siddiq M (2010) J Appl Polym Sci 118:3324–3332
Khan A, Farooqi ZH, Siddiq M (2012) J Appl Polym Sci 124:951–957
Kelarakis A, Mai SM, Havredaki V, Nace VM, Booth C (2001) Phys Chem Chem Phys 3:4037–4043
Maskos M (2006) Polymer 47:1172–1178
Tattershall CE, Jerome NP, Budd PM (2001) J Mater Chem 11:2979–2984
Tattershall CE, Aslam SJ, Budd PM (2002) J Mater Chem 12:2286–2291
Khan A, Siddiq M (2013) J Polym Res 20:1–9
Usman M, Siddiq M (2013) J Chem Thermodyn 58:359–366
Cheema MA, Taboada P, Barbosa S, Castro E, Siddiq M, Mosquera V (2008) J Chem Thermodyn 40:298–308
Tanford C (1980) The hydrophobic effect. Wiley, New York
William RJ, Phillips JN, Mysels KJ (1955) Trans Faraday Soc 51:561–569
Sultana SB, Bhat SGT, Rakshit AK (1997) Langmuir 13:4562–4568
Provencher SW (1979) Makromol Chem 180:201–209
Chaibundit C, Ricardo NMPS, Crothers M, Booth C (2002) Langmuir 18:4277–4283
Barbosa S, Cheema MA, Taboada P, Mosquera V (2007) J Phys Chem B 111:10920–10928
Wu C, Xia KQ (1994) Rev Sci Instrum 65:587–590
Vrij A (1978) J Chem Phys 69:1742–1747
Mai SM, Booth C, Nace VM (1997) Eur Polym J 33:991–996
Siddiq M, Harrison W, Tattershall CE, Budd PM (2003) Phys Chem Chem Phys 5:3968–3972
Siddiq M, Liu G, Zhang G, Khan A, Budd PM (2010) Polym Bull 65:521–531
Acknowledgments
We are highly grateful to Dr. Carin Tattershall (University of Manchester) for synthesizing the dimethylamino- and trimethylammonium-tipped diblock copolymers. We are also thankful to Prof. Peter M. Budd of the University of Manchester for helpful discussions. Dr. Abbas Khan is grateful to the Higher Education Commission, H.E.C., Pakistan for financial support under the indigenous Ph.D. fellowship scheme. He also wishes to acknowledge the Academy of Sciences for Developing Countries for a split Ph.D. research fellowship to work in the Department of Chemical Physics, University of Science and Technology, Hefei, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khan, A., Siddiq, M. Effect of end-group modification, hydrophilic/hydrophobic block ratio and temperature on the surface, associative and thermodynamic behaviour of poly(ethylene oxide)-b-poly(butylene oxide) diblock copolymers in aqueous media. J Polym Res 21, 560 (2014). https://doi.org/10.1007/s10965-014-0560-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-014-0560-y