Abstract
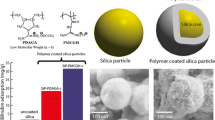
The effect of polycationic polymers of polyacrylate guanidine (PAG) and polymethacrylate guanidine (PMAG) on bilirubin absorbance were studied in phosphate buffer (pH 7.4). It was shown that the change in absorbance spectra of bilirubin in the presence of PAG/PMAG can be associated with the formation of a bilirubin-polymer complex and dissociation of tetramers on bilirubin monomers. Also, the organic-inorganic composite materials based on silica gels and guanidine polymers were synthesized via the sol-gel technique. The incorporated guanidine polymers have a big influence on particle size distribution of silica gel due to their high cross-linking ability. The infrared spectroscopy revealed the presence of guanidine polymers inside solid networks of silica gel. The bilirubin adsorption process onto a guanidine functionalized silica surface was investigated. The Langmuir and Redlich-Peterson isotherm models were tested to explain the adsorption mechanism. The analysis of the adsorption isotherms confirms the possibility of electrostatic interactions of bilirubin molecules with guanidine polymers incorporated inside silica matrix. We conclude that cationic guanidine polymers might be effectively applied for bilirubin removal.







Similar content being viewed by others
References
Tillet G, Boutevin B, Ameduri B (2011) Prog Polym Sci 36:191–217
Siedenbiedel F, Tiller JC (2012) Polymers 4:46–71
Wang X, McCord MG (2007) J Appl Polym Sci 104(6):3614
Tew GN, Scott RW, Klein ML, De Novo WF (2010) Acc Chem Res 43:30–39
Kenawy ER, Worley SD, Broughton R (2007) Biomacromolecules 8:1359–1384
Tiller JC, Lee SB, Lewis K, Klibanov AM (2002) Biotechnol Bioeng 79:465–471
Timifeeva L, Kleshcheva N (2007) Appl Microbiol Biotechnol 89:475–492
Banerjee I, Pangule RC, Kane RS (2011) Adv Mater 23:690–718
Gonzales FP, Maisch T (2010) Drug News Perspect 23:167–174
Gabriel GJ, Som A, Madkour AE, Eren T, Tew GN (2007) Mater Sci Eng R 57:28–64
Lienkamp K, Madkour AE, Musate A, Nelson CF (2008) J Am Chem Soc 130:9836–9843
Funhoff AM, Nostrum CF, Lok MC (2004) Bioconjug Chem 15:1212–1220
Timin AS, Rumyantsev EV (2013) Res Chem Intermed. doi:10.1007/s11164-013-1361-3
Carlos PM et al (2013) Biomater Sci 1:736–744
Qian L, Dong C et al (2013) Holzforschung. doi:10.1515/hf-2012-0206
Kratzer C, Tobudic S, Macfelda K (2007) Antimicrob Agents Chemother 51(9):3437–3439
Stelmakh SA, Grigoreva MN (2012) J Mater Sci Eng B 2(8):421–428
Treat NJ, Smith D, Tenq C et al (2012) ACS Macro Lett 1:100–104
Zunszain PA, Ghuman J, McDonagh AF, Curry S (2008) J Mol Biol 381:394–406
Baydemir G, Andac M, Bereli N et al (2007) Ind Eng Chem 46:2843–2852
Lee KH, Wendon J, Lee M (2002) Liver Transplant 8:591–602
Mukerjee P, Ostrow JD (2010) Biochemisty 11:16–28
Kim Y, Binauld S, Stenzel MH (2012) Biomacromolecules 13(10):3418–3426
Menaa B, Menaa F, Aiolfi-Guimaraes C, Sharts O (2010) Int J Nanotechnol 7:1–45
Dabrowski A, Barczak A, Dudarko OA (2007) Pol J Chem 81:475–483
El-Nahhal IM, El-Ashgar NM (2007) J Organomet Chem 692:2861–2870
Li XG, Ma XL, Sun J, Huang MR (2009) Langmuir 25:1675–1684
Dudarko OA, Zub YL, Dabrowski A (2011) Glas Phys Chem 6:596–602
Lynch I, Dawson K (2008) Nano Today 3:40–47
Mansur HS, Orefice RL, Vasconcelos WL, Lobato ZP, Machardo LJC (2005) J Mater Sci Mater Med 16:333–340
Timin AS, Rumyantsev EV (2013) J Sol-Gel Sci Technol 67:297–303
Sivov NA, Khashirova SY (2008) Mod Tendencies Org Bioorg Chem 27:310–335
Rai AK, Rai SB, Rai DK, Singh VB (2002) Spectrochim Acta A 58:2145–2152
Sugiol S, Kashima A, Mochizuki S, Noda M, Kobayashi K (1999) Protein Eng 12:439–446
Xu Y, Axe L (2005) J Colloid Interface Sci 282:11–19
Shengju W, Li Fengting X, Ran WS, Guangtao L (2010) J Nanoparticle Res 12:2111–2124
Mansur HS, Lobato ZP, Orefice RL, Vansconcelos WL (2001) Adsorption 7:105
Atieh MA, Bakather OY, Al-Tawbini B, Alaadin A et al (2010) Bioinorg Chem Appl. doi:10.1155/2010/603978
Langmuir I (1918) J Am Chem Soc 40:1361–1403
Kumar KV, Porkodi K (2006) J Hazard Mater 138:633–635
Hamdaoui O, Naffrechoux E (2007) J Hazard Mater 147:401–411
Acknowledgments
We thank Dr. Khashirova S. Yu., the Department of Macromolecular Compounds, Kabardino-Balkar State University by N.M. Berbecova, for the synthesis of guanidine polymers which were used in this work. The work was supported by a grant of the RFBR (project No. 12-03-31309), bursary of the President of the Russian Federation No. SP- for young scientists and graduate students engaged in advanced research and development in priority directions of modernization of the Russian economy (2013–2015) and a grant of the President of the Russian Federation No. МК-287.2014.3 (2014–2015).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Timin, A.S., Solomonov, A.V. & Rumyantsev, E.V. Polyacrylate guanidine and polymethacrylate guanidine as novel cationic polymers for effective bilirubin binding. J Polym Res 21, 400 (2014). https://doi.org/10.1007/s10965-014-0400-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-014-0400-0