Abstract
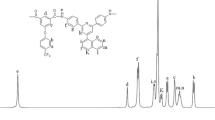
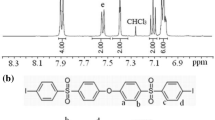
Several series of alternate poly(amide-imide)s [P(A-alt-I)s] were synthesized by aromatic dicarboxylic acid (I- p or I- m), which was prepared by the condensation of p-phenylenediamine (or m-phenylenediamine), trimellitic anhydride, and various aromatic diamines by means of direct polycondensation. A diimide-diacid (I- p) with a p-phenylene group was used to synthesize P(A-alt-I)s III, and P(A-alt-I)s IV were synthesized by a diimide-diacid (I- m) prepared from m-phenylenediamine. Another series of P(A-alt-I)s V was synthesized from both I- p and I- m (1/1 mole) with various diamines. Polymers of series III have low inherent viscosities and limited solubility, but polymers of series IV have high degrees of polymerization. Series V copolycondensated from I- p and I- m has improved solubility and degrees of polymerization relative to series III. The degree of crystallinity was found to be III > V > IV. Glass transition temperatures for most of series III were not observed below 400 °C, and those of series IV and V were in the range of 238–325 °C and 262–328 °C, respectively. The 10% weight loss temperatures in nitrogen or in air of these three series are all in the range of 482–582 °C. Because series V has limited solubility for casting into films from DMAc solutions, two diamines were selected to synthesize series VI by changing the I- p/I- m ratio. Solubility was improved when the content of I- p in diimide-diacid was less than 15%, and the degree of crystallinity reduced as the content of I- p in diimide-diacid decreased. Polymers containing a few I- p showed an increase in the initial modulus.
Similar content being viewed by others
References
K. L. Mittal, Ed., Polyimides: Synthesis, Characterization and Application, Vols. 1 and 2, Plenum Press, New York, 1984.
C. Feger, M. M. Khojasteh and J. E. McGrath, Eds., Polyimides: Materials, Chemistry and Characterization, Elsevier, Amsterdam, 1989.
D. Wilson, H. D. Stenzenberg and P. M. Hergenrother, Eds., Polyimides, Chapman and Hall, New York, 1990.
M. J. M. Abadie and B. Sillion, Eds., Polyimides and Other High-Temperature Polymers, Elsevier, Amsterdam, 1991.
C. Feger, M. M. Khojasteh and M. S. Htoo, Eds., Advances in Polyimide Science and Technology, Technomic, Lancaster, 1993.
P. E. Cassidy, Thermally Stable Polymers, Dekker, New York, 1980.
H. H. Yang, in Aromatic High-Strength Fibers, Wiley, New York, 1989.
Y. Imai, N. N. Malder and M. Kakimoto, J. Polym. Sci., Polym. Chem., 22, 2189 (1984).
Y. Imai and M. Kakimoto, Polym. Plast. Technol. Eng., 28, 371 (1989).
C. P. Yang and W. T. Chen, Macromolecules, 26, 4865 (1993).
M. Bruma, F. Mercer, J. Fitch and P. Cassidy, J. Appl. Polym. Sci., 56, 527 (1995).
J. H. Lin and C. P. Yang, Macromol. Chem. Phys., 197, 529 (1996).
Y. T. Chern and W. L. Wang, J. Polym. Sci., Polym. Chem., 34, 1501 (1996).
G. C. Eastmond, J. Paprotny and R. S. Irwin, Macromolecules, 29, 1382 (1996).
F. Akutsu, H. Hayashi, M. Miura and K. Nagakubo, Makromol. Chem., Rapid Commun., 6, 407 (1985).
F. Akutsu, A. Suzuki, F. Saitoh, K. Naruchi, M. Miura and K. Nagakubo, Makromol. Chem., 188, 1253 (1987).
F. Akutsu, T. Kataoka, K. Naruchi, M. Miura and K. Nagakubo, Polymer, 28, 1787 (1987).
Y. Kasashima, K. Yamamoto, N. Ando, F. Akutsu, K. Naruchi and M. Miura, Polym. J., 26, 1298 (1994).
Y. Kasashima, H. Kumada, K. Yamamoto, F. Akutsu, K. Naruchi and M. Miura, Polymer, 36, 645 (1995).
F. Akutsu, N. Sugiyama, N. Ando, Y. Kasashima, M. Inoki and K. Naruchi, Polym. J., 27, 1025 (1995).
S. S. Ray, A. K. Kundu, M. Maiti, M. Ghosh and S. Maiti, Angew. Makromol. Chem., 122, 153 (1984).
S. S. Ray, A. K. Kundu, M. Ghosh and S. Maiti, J. Polym. Sci., Polym. Chem., 24, 603 (1986).
M. Ghosh, Angew. Makromol. Chem., 172, 165 (1989).
C. P. Yang, S. H. Hsiao and J. H. Lin, U.S. Pat. 5,268,487 (1993).
C. P. Yang, S. H. Hsiao and J. H. Lin, Angew. Makromol. Chem., 193, 1299 (1992).
C. P. Yang and W. T. Chen, J. Polym. Sci., Polym. Chem., 32, 435 (1994).
C. P. Yang and W. T. Chen, J. Polym. Sci., Polym. Chem., 32, 1101 (1994).
C. P. Yang and J. H. Lin, J. Polym. Sci., Polym. Chem., 32, 2653 (1994).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, RS., Yang, CP. Alternate poly(amide-imide)s based on 1,4-and 1,3-bis(4-trimellitimido)benzene and their mixtures. J Polym Res 5, 233–241 (1998). https://doi.org/10.1007/s10965-006-0062-7
Issue Date:
DOI: https://doi.org/10.1007/s10965-006-0062-7