Abstract
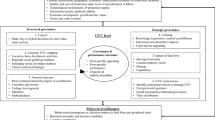
In the last 20 years, state governments have funded a large number of science-based economic development programs. Remarkably, there have been few evaluations of either their economic impact or their prospects for economic impact. The 2004 decision by California voters to issue bonds to fund $3 billion of research on human pluripotent stem cells is an ideal case study for introducing an evaluation methodology for the progress by state programs in developing and transferring research results. In the process of evaluating the California stem cell initiative, we make three methodological contributions to the study of the impacts of sub-national science-based economic development programs. First, the following data sources are introduced as indicators of the prospective economic benefits of these programs: changes in targeted federal research funding, patenting, small business investment research grants, venture capital investment, new firm formations, and clinical trials. Second, the use of regional or industrial controls is introduced. In the case study of the California stem cell initiative the regional control is the comparison of the two dominant California stem cell research and firm clusters, San Diego and the San Francisco Bay Area, to the other dominant cluster, Boston. The biotechnology industry, as a whole, is used to control for exogenous shocks such as changes in the overall interest in new life science technologies. Third, because technology transfer is predicated upon commercialization, a value chain perspective can visualize commercialization obstacles. In this study, a stylized human stem cell therapy value chain is compared to the existing biotechnology value chain and the differences suggest areas where human stem cell therapies are likely to face difficulties. The discussion and conclusion evaluates the progress of the California stem cell initiative and suggests that the evaluation methodology developed can improve the evaluation and guidance of science-based economic development programs.

Source Analysis by University of Bordeaux GREThA researchers, July 2015

Source University of Bordeaux GREThA, July 2015

Source SBIR.gov, accessed March 9, 2015

Source SBIR.gov, accessed March 9, 2015

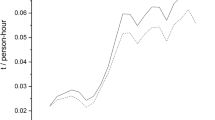
Source Thomson VentureXpert

Source Thomson VentureXpert and authors’ data

Source Thomson VentureXpert and authors’ data

Source U.S. Clinical Trial Registry

Similar content being viewed by others
Notes
Hegde and Sampat (2015) show that in the case of orphan diseases that the influence of advocates exists but did not have a large impact on research choices.
The passage of Proposition 71 led to a major burst of philanthropic funding of SC facilities in California (Walshok and Shapiro 2015).
Federal statistics report SBIR grants by department, and the NIH is in the Department of Health and Human Services (HHS). Fortunately, NIH funding comprises nearly all HHS research funding. The SC SBIR grant data includes individual firms only once, though many firms receive multiple grants. In the HHS data, due to the enormous number, these are simple counts not curated to include each firm only once. For this reason, the data are not strictly comparable. Due to the large number, this should have little impact on the aggregate comparisons.
Oddly enough, the CIRM gave the grant to Caladrius, although it was a New York firm that had opened a branch in Irvine, CA (Caladrius Biosciences 2015).
References
Adelson, J. W., & Weinberg, J. K. (2010). The California SC initiative: Persuasion, politics, and public science. American Journal of Public Health, 100(3), 446–451.
Alberta, H. B., Cheng, A., Jackson, E. L., Pjecha, M., & Levine, A. D. (2015). Assessing state SC programs in the United States: How has state funding affected publication trends? Cell SC, 16(2), 115–118.
Audretsch, D. B., Link, A. N., & Scott, J. T. (2002). Public/private technology partnerships: Evaluating SBIR-supported research. Research Policy, 31(1), 145–158.
Baker, L., & Deal, B. (2004). Economic impact analysis: Proposition 71 California stem cell research and cures initiative. Analysis Group, September, 14, 2004.
Benjamin, R. (2013). People’s science: Bodies and rights on the SC frontier. Palo Alto, CA: Stanford University Press.
Brown, N. (2003). Hope against hype: Accountability in biopasts, presents and futures. Science Studies, 16(2), 3–21.
Bubela, T., Li, M. D., Hafez, M., Bieber, M., & Atkins, H. (2012). Is belief larger than fact: Expectations, optimism and reality for translational SC research. BMC Medicine, 10(1), 133–156.
Buxton, M. (2011). The payback of ‘payback’: Challenges in assessing research impact. Research Evaluation, 20(3), 259–260.
Caladrius Biosciences. (2015). Caladrius Biosciences, Inc. finalizes corporate name change from NeoStem, Inc. http://www.caladrius.com/press-release/caladrius-biosciences-inc-finalizes-corporate-name-change-from-neostem-inc/.
Casper, S. (2014). The University of California and the evolution of the biotechnology in San Diego and San Francisco. In M. Kenney & D. Mowery (Eds.), Public universities and regional development: Insights from the University of California (pp. 66–96). Stanford: Stanford University Press.
Casper, S. (2015). Building research capacity for SC leadership in California. Powerpoint Ppresentation, Keck Graduate Institute, Claremont Colleges (July 22).
Cohen, W. M., Nelson, R. R., & Walsh, J. P. (2002). Links and impacts: The influence of public research on industrial R&D. Management Science, 48(1), 1–23.
Cohen, L. R., & Noll, R. G. (2002). The technology pork Barrel. Washington, DC: Brookings Institution Press.
Cooke, P. (2004). Life sciences clusters and regional science policy. Urban Studies, 41(5–6), 1113–1131.
Dietz, J. S., & Rogers, J. D. (2012). Meanings and policy implications of “transformative research”: Frontiers, hot science, evolution, and investment risk. Minerva, 50(1), 21–44.
Dodson, B. P., & Levine, A. D. (2015). Challenges in the translation and commercialization of cell therapies. BMC Biotechnology, 15(1), 70–85.
Feldman, M. P., Lanahan, L., & Lendel, I. (2013). Experiments in the laboratories of democracy: State scientific capacity building. Economic Development Quarterly, 28(2), 107–131.
Feller, I. (2013). Performance measures as forms of evidence for science and technology policy decisions. Journal of Technology Transfer, 38(5), 565–576.
Gallicano, G. I. (2010). SCs: Past, present, and future. www.asgct.org/am10/program/…/Session_124_-_1_gallicano.pdf. Accessed 18 Aug 2015.
Garde, D. (2013). Geron unloads its SC coffers to BioTime in stock deal. Fierce Biotech (October 2013). http://www.fiercebiotech.com/financials/geron-unloads-its-stem-cell-coffers-to-biotime-stock-deal/.
Geels, F., & Smit, W. (2000). Failed technology futures: Pitfalls and lessons from a historical survey. Futures, 32(9/10), 867–885.
Geiger, R. L., & Sá, C. (2005). Beyond technology transfer: US state policies to harness university research for economic development. Minerva, 43(1), 1–21.
Gereffi, G., Humphrey, J., & Sturgeon, T. (2005). The governance of global value chains. Review of International Political Economy, 12(1), 78–104.
Gilbert, R. J. (2006). Dollars for genes: Revenues generation by the California Institute for Regeneration Medicine. Berkeley Technology Law Journal, 21, 1107–1142.
Goldstein, L. S. (2010). Unconventional allies: Interdisciplinary approaches to science policy and funding. Trends in Cell Biology, 20(12), 695–698.
Goldstein, L. S. (2011). In the trenches: Lessons for scientists from California’s Proposition 71 campaign. Molecular Biology of the Cell, 22(21), 3943–3944.
Griliches, Z. (1979). Issues in assessing the contribution of research and development to productivity growth. Bell Journal of Economics, 10(Spring), 92–116.
Hargadon, A. B., & Douglas, Y. (2001). When innovations meet institutions: Edison and the design of the electric light. Administrative Science Quarterly, 46(3), 476–501.
Heathman, T. R., Nienow, A. W., McCall, M. J., Coopman, K., Kara, B., & Hewitt, C. J. (2015). The translation of cell-based therapies: Clinical landscape and manufacturing challenges. Regenerative Medicine, 10(1), 49–64.
Hegde, D., & Sampat, B. (2015). Can private money buy public science? Disease group lobbying and federal funding for biomedical research. Management Science, 61(10), 2281–2298.
Jain, S., & George, G. (2007). Technology transfer offices as institutional entrepreneurs: The case of Wisconsin Alumni Research Foundation and human embryonic SCs. Industrial and Corporate Change, 16(4), 535–567.
Jones, C. I., & Williams, J. C. (1998). Measuring the social return to R&D. Quarterly Journal of Economics, 113(4), 1119–1135.
Kapoor, R., & Klueter, T. (2015). Decoding the adaptability–rigidity puzzle: Evidence from pharmaceutical incumbents’ pursuit of gene therapy and monoclonal antibodies. Academy of Management Journal, 58(4), 1180–1207.
Kenney, M. (1986). Biotechnology: The University-industrial complex. New Haven: Yale University Press.
Kneller, R. (2010). The importance of new companies for drug discovery: Origins of a decade of new drugs. Nature Reviews Drug Discovery, 9(11), 867–882.
Knoepfler, P. S. (2015). From bench to FDA to bedside: US regulatory trends for new SC therapies. Advanced Drug Delivery Reviews, 82, 192–196.
Lerner, J. (1999). The government as venture capitalist: The long-run impact of the SBIR program. Journal of Private Equity, 72(3), 55–78.
Lewenstein, B. V. (1995). From fax to facts: Communication in the cold fusion saga. Social Studies of Science, 25(3), 403–436.
Li, M. D., Atkins, H., & Bubela, T. (2014). The global landscape of SC clinical trials. Regenerative Medicine, 9(1), 27–39.
Lichtenberg, F. R., & Siegel, D. S. (1991). The impact of R&D investment on productivity-new evidence using linked R&D-LRD data. Economic Inquiry, 29, 203–228.
Longaker, M. T., Baker, L. C., & Greely, H. T. (2007). Proposition 71 and CIRM—assessing the return on investment. Nature Biotechnology, 25(5), 513–521.
Mansfield, E. (1980). Basic research and productivity increase in manufacturing. American Economic Review, 70, 863–873.
Mansfield, E. (1991). Academic research and industrial innovation. Research Policy, 20, 1–12.
Murray, F. (2007). The stem-cell market-patents and the pursuit of scientific progress. New England Journal of Medicine, 356(23), 2341–2343.
National Institutes of Health. (2015). NIH Project Reporter. https://projectreporter.nih.gov/reporter.cfm.
Nelson, R. R. (1959). The simple economics of basic scientific research. Journal of Political Economy, 67(3), 297–306.
NeoStem. (2007). Prospectus 424B1 filed July 17, 2007. https://www.sec.gov/Archives/edgar/data/320017/000110465907054357/a07-13864_1424b1.htm.
Nerem, R. M. (2010). Regenerative medicine: The emergence of an industry. Journal of the Royal Society, Interface, 7(supplement 6), S771–S775.
Noll, R. G. (2006). Designing an effective program of state-sponsored human embryonic SC research. Berkeley Technology Law Journal, 21, 1143–1176.
Padgett, J. F., & Powell, W. W. (2012). The emergence of organizations and markets. Princeton: Princeton University Press.
Perkmann, M., Fini, R., Ross, J. M., Salter, A., Silvestri, C., & Tartari, V. (2015). Accounting for universities’ impact: Using augmented data to measure academic engagement and commercialization by academic scientists. Research Evaluation, 24(4), 380–391.
Powell, W. W., Koput, K. W., Bowie, J. I., & Smith-Doerr, L. (2002). The spatial clustering of science and capital: Accounting for biotech firm-venture capital relationships. Regional Studies, 36(3), 291–305.
Rafols, I., Hopkins, M. M., Hoekman, J., Siepel, J., O’Hare, A., Perianes-Rodríguez, A., et al. (2014). Big pharma, little science? A bibliometric perspective on Big Pharma’s R&D decline. Technological Forecasting and Social Change, 81, 22–38.
Rao, M. S. (2011). Funding translational work in cell-based therapy. Cell SC, 9(1), 7–10.
Rosenbloom, J. L. (2007). The geography of innovation commercialization in the United States during the 1990s. Economic Development Quarterly, 21(1), 3–16.
Rothaermel, F. T., & Deeds, D. L. (2004). Exploration and exploitation alliances in biotechnology: A system of new product development. Strategic Management Journal, 25(3), 201–221.
SHEEO (State Higher Education Executive Officers). (2016). State Higher Education Finance, FY 2015.
Strickland, S. P. (1972). Politics, science, and dread disease: A short history of United States medical research policy. Cambridge: Harvard University Press.
Teece, D. J. (1986). Profiting from technological innovation: Implications for integration, collaboration, licensing and public policy. Research Policy, 15(6), 285–305.
Thursby, J. G., Jensen, R., & Thursby, M. C. (2001). Objectives, characteristics and outcomes of university licensing: A survey of major US universities. Journal of Technology Transfer, 26(1–2), 59–72.
Toole, A. A., & Czarnitzki, D. (2007). Biomedical academic entrepreneurship through the SBIR program. Journal of Economic Behavior & Organization, 63(4), 716–738.
Turner, L., & Knoepfler, P. (2016). Selling SCs in the USA: Assessing the direct-to-consumer industry. Cell SC, 19, 1–2.
Wallace, M. L., & Rafols, I. (2015). Research portfolio analysis in science policy: Moving from financial returns to societal benefits. Minerva, 53(2), 89–115.
Walshok, M., & Shapiro, J. (2015). Private communication (July 22, 2015).
Zhang, J., & Patel, N. (2005). The dynamics of California’s biotechnology industry. Sacramento: Public Policy Institute of California.
Acknowledgements
The authors acknowledge partial financial support from Americans for the Cure. We acknowledge the helpful comments of Steven Casper, Al Link, Josh Shapiro, Mary Walshok and one anonymous reviewer. However, all the arguments, analysis, and conclusions are entirely the responsibility of the authors, and none of them should, in any way, be attributed to the sponsors or commentators acknowledged here.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kenney, M., Patton, D. Sub-national technology policy and commerce: evaluating the impacts of the California Institute for Regenerative Medicine. J Technol Transf 43, 47–68 (2018). https://doi.org/10.1007/s10961-017-9580-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10961-017-9580-1
Keywords
- Stem cells
- Regenerative medicine
- California Institute for Regenerative Medicine
- Evaluation
- Commercialization
- Research goal setting