Abstract
The heat capacity of different mixtures of terpenes (α-pinene, β-pinene, limonene oxide) and solvents (acetone, toluene, ethyl acetate) at atmospheric pressure (85.1 kPa atm in Medellin, Colombia) were measured using a microcalorimeter at several terpene molar fractions and from room temperature to a value close to the solvent boiling point. The mixtures analyzed were acetone + α-pinene from 298.15 to 323.15 K, toluene + limonene oxide and toluene + β-pinene from 298.15 to 358.15 K, and ethyl acetate + β-pinene between 298.15 and 338.15 K. These mixtures, at the selected temperature ranges, are used in fine chemical catalytic reactions. The experimental heat capacity values were fitted to polynomials as a function of temperature. Excess heat capacity was calculated with the measured molar heat capacity for all the mixtures, it decreased with temperature. Experimental uncertainty was less than 1.5% with a confidence level of 95% using k = 2. The experimental results were consistent, for example the heat capacity of ethyl acetate + β-pinene mixture increased as the temperature increased and decreased with the composition of the solvent; at 308.15 K the heat capacity decreased from 252.73 to 245.17 J mol−1 K−1 when solvent composition increased from 0.1546 to 0.2797 and at a solvent composition of 0.1546, heat capacity increased from 237.26 to 252.73 J·mol−1·K−1 when temperature increased from 298.15 to 308.15 K.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In organic reactions it is common to use solvents that favor certain reaction conditions such as conversion or selectivity. Furthermore, they facilitate the transfer of heat, solubilization of reagents and in catalytic reactions they favored the regioselectivity of the reaction and catalyst activity [1]. The correct choice of a solvent is important in fine chemical reactions; therefore it is important to know details of the interaction it has with the reagent. One of the properties that is important to measure is the heat capacity of mixtures. This property is useful in the design of chemical process plants and in the thermodynamic analysis of the processes. Due to the use of terpenes in several processes, such as oil extraction and reagent purification, there is literature available on heat capacities of terpenes mixtures or terpene-solvent mixtures. Some of the studied mixtures are: α-pinene + β-pinene mixture between 313.15 and 418.15 K at atmospheric pressure [2]; 1,8 cineole + ethanol mixture [3] from 304.7 to 324.5 K; p-cymene + ethanol mixture at atmospheric pressure varying composition of the ethanol between a molar fraction of 0 to 1 and temperature (298.6–328.4 K) [4].
The study of properties of mixtures is of great importance as most of the compounds in nature and chemical processes from mixtures. Turpentine is composed mainly by α- and β-pinene, among other terpenes, and it is used in reactions such as the pyrolysis of β-pinene to produce myrcene [5] or in its direct hydration to produce α-terpineol [6]. The study of the heat capacity of mixtures containing terpenes could constitute a basis for the optimization of fine chemical reactions. Some cases of terpene transformation in presence of a solvent include the oxidation of α-pinene and limonene using acetone to produce verbenone and carvone, respectively [7]. The Prins condensation of paraformaldehyde and β-pinene in toluene to produce nopol [8] or in ethyl acetate that increases the reaction rate [9], and the isomerization of α-pinene epoxide and limonene epoxide in toluene, where the solvent has an effect on campomelic aldehyde selectivity [10]. Understanding the solvent-substrate interactions contributes to a deeper understanding of the reaction, allowing the researcher to select the ideal solvent to achieve high yields.
In mixtures, it is important to calculate the excess heat capacity to know about the deviations from ideal substrate-solvent interactions. These deviations are associated to variations in the internal energy between pure substances and mixtures. Fujisawa et al. [11] reported excess molar heat capacities of (R)-( +)-α-pinene + (S)-(–)-α-pinene mixture, noting that there were no significant variations between the heat capacities of the isomers. The Dortmund Data Bank reports heat capacities of pure substances and mixtures, as well as excess heat capacities; however, there were few reported systems of excess heat capacities of mixture of terpenes or terpenes with other compounds (α-pinene + β-pinene; D-α-pinene + L-α-pinene, D-( +)-limonene + L-(−)-limonene; ethanol + carvacrol).
The present work reports the experimental evaluation of the heat capacity of various solvent + terpene mixtures (acetone + α-pinene; ethyl acetate + β-pinene; toluene + limonene oxide and toluene + β-pinene) that are used in catalytic transformations, and their variation with temperature and molar fraction. Furthermore, the excess heat capacity of these mixtures as a function of temperature and solvent composition is reported.
2 Experimental
2.1 Materials
Table 1 shows information about the reagents employed in the analysis.
2.2 Measurement of Heat Capacity
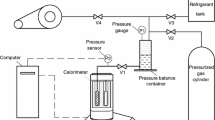
The heat capacity was determined using a microcalorimeter Setaram E/µDSC 7 Evo-1A, and the mass of the samples was weighed using a Sartorius BP2105 balance with an uncertainty of ± 0.1 mg. The microcalorimeter used the technique of differential scanning calorimetry with two standards cells of 1 cm3 settled in parallel, first, a background was run with the two empty cells, then the substance of interest was placed in the sample cell and the reference cell was kept empty. Calibration was carried out by supplier following the reported procedure in [12], the calibration was carried out with standard naphthalene, Table S1 in Supplementary Information shows the experimental and reported values, the calibration result indicated a background noise lower than 0.3 μW. As a reference the heat capacity of water was obtained in this research and was compared with literature data, the results are shown in Table S2 in Supplementary Information. Reproducibility of measurements was evaluated with the average of three measurements of β-pinene heat capacity and the uncertainty of the heat capacity was calculated with the different relative standard deviation using scan method, obtaining a value lower than 2% with a confidence level of 95% using a k factor equal to 2 [13, 14].
The heat capacity of the mixtures was obtained with a heating rate of 1 K·min−1, followed by an isothermal delay of 1800 s and a sample amount of 100 ± 0.1 mg. The mole fraction of the solvent was varied between 0 and 1 and its uncertainty was estimated to be u(x1) = ± 0.0005, considering the uncertainty of the balance. The temperature range used in the analyses depended on each solvent: acetone (293.15–323.15) K, toluene (293.15–363.15) K, and ethyl acetate and acetonitrile (293.15–343.15) K. A trendline was fitted to the data to find an equation that represented the heat capacity as a function of solvent molar fraction at different temperatures.
For all the mixtures, a cubical polynomial function was obtained for the heat capacity as a function of the molar fraction of the solvent at constant temperature, Eq. 1.
where CpM is the heat capacity of the mixture in J·mol−1·K−1 and x1 represents the molar fraction of the solvent in the mixture. The values were obtained at different temperatures, and A, B, C and D are the parameters of the model.
2.3 Measurement of Excess Heat Capacity
The excess heat capacity was obtained for each mixture at different temperatures using Eq. 2 [3].
where CpM is in J·mol-1·K-1 and corresponds to the heat capacity of the mixture Cpi is the heat capacity of pure compound i, and xi is the molar fraction of the component i, where solvent is i = 1 and terpene is i = 2.
3 Results and Discussion
The heat capacity associated to pure compounds (solvents, α-pinene and β-pinene) was compared with data from literature to analyze the data reliability; the comparison is presented in Table 2.
The isobaric heat capacity of acetone, α-pinene, β-pinene, ethyl acetate and toluene obtained at different temperatures was compared with reported data in Figs. 1, 2, 3, 4, 5. In general, the experimental results and the literature presented the same tendencies with an increase in heat capacity as the temperature increases, with a deviation not higher than 5%, except in the case of toluene at temperatures close to the boiling point. The differences between the values could be attributed to the equipment and the pressure conditions used in the analysis.
a Isobaric molar heat capacity for acetone: (■ -) this work; (★) Low and Moelwyn-Hughes [16]; (▲) Yaws [17]; (●) Malhotra and Woolf [15]. b Percentage relative deviations of experimental molar heat capacity of acetone obtained in this work compared to reported values in literature: (★) Low & Moelwyn-Hughes [16]; (▲) Yaws [17]; (●) Malhotra and Woolf [15]
a Isobaric molar heat capacity for α-pinene: (■ -) this work; (★) Langa et al. [2]; (▲) Fujisawa et al. [11]; (●) Sampaio & Nieto [24]. b Percentage relative deviations of experimental molar heat capacity of α-pinene obtained in this work compared to reported values in literature: (★) Langa et al. [2]; (▲) Fujisawa et al. [11]; (●) Sampaio & Nieto [24]
a Isobaric molar heat capacity for β-pinene: (■ -) this work; (★) Langa et al. [2]; (●) Sampaio & Nieto [24]. b Percentage relative deviations of experimental molar heat capacity of β-pinene obtained in this work compared to reported values in literature: (★) Langa et al. [2]; (●) Sampaio & Nieto [24]
a Isobaric molar heat capacity for ethyl acetate: (■ -) this work; (★) Yaws. [17]; (●) Zabransky et al. [20]. b Percentage relative deviations of experimental molar heat capacity of ethyl acetate obtained in this work compared to reported values in literature: (★) Yaws [17]; (●) Zabransky et al. [20]
a Isobaric molar heat capacity for toluene: (■ -) this work; (★) Sampaio & Nieto [24]; (▲) Pedersen, Kay, et al. [22]; (●) Paramo et al. [23]. b Percentage relative deviations of experimental molar heat capacity of toluene obtained in this work compared to reported values in literature: (★) Sampaio & Nieto [24]; (▲) Pedersen, Kay, et al. [22]; (●) Paramo et al. [23]
For each of the analyzed mixtures, the molar heat capacity evaluated as a function of temperature, are reported in Tables S3 to S6 in Supplementary Information, adjusting the data to a polynomial equation. The constants of the adjusted equations are detailed later in the text at different temperatures for toluene + β-pinene mixture in Table 3, for acetone + α-pinene mixture in Table 4, for toluene + limonene oxide mixture in Table 5, and for ethyl acetate + β-pinene mixture in Table 6. For all the mixtures, the molar heat capacity increases with temperature and terpene concentration. In general, the heat capacity of the terpenes presents higher variations with temperature than it does with the molar fraction of the solvent.
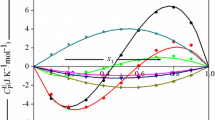
The excess heat capacity was obtained for the pure substances using Eq. 2. Figure 6 shows the data of heat capacity as a function of temperature and solvent composition for toluene + β-pinene mixture, Fig. 7 shows the excess heat capacity for the same mixture. Following, Fig. 8 and Fig. 9 correspond to acetone + α-pinene mixture, Fig. 10 and Fig. 11 correspond to toluene + limonene oxide mixture, and Fig. 12 and Fig. 13 to ethyl acetate + β-pinene mixture. The obtained excess heat capacity values were of the same order of magnitude as for other reported solvent + terpene systems, such as ethanol + 1,8 cineole or ethanol + p-cymene mixtures [3, 4]. The excess heat capacity has a bell-like behavior, with the maximum located in the region of the largest amount of solvent, all of which is affected by the interactions between the substances involved. In general terms, the molar heat capacity of the mixture presents greater variations with change in the molar fraction of solvent than it does with change in temperature. The excess heat capacity has the highest peak in the 0.5 ≤ x1 ≤ 0.7 range.
When analyzing the different mixtures, it is observed that the excess heat capacity decreases with temperature, except in the case of the acetone + α-pinene mixture, that has small variations within the uncertainty range. With respect to the other samples, a decrease with temperature increase is observed, although the excess heat capacity usually increases with temperature. This behavior has also reported in mixtures such as ( +)-α-pinene + (S)-(-)-α-pinene mixture [11] and 1-butanol + toluene [25].
4 Conclusions
The excess heat capacities and heat capacities of four mixtures of terpenes and solvents of interest in fine chemical reactions were measured in liquid phase over a wide temperature range. The relationship between heat capacity and solvent composition, for a given temperature, was fitted by a third order polynomial. The obtained data trends were as expected, a bell shape for the excess heat capacity, and an increase of the heat capacity respect to temperature increase, with higher values for the terpenes than for the solvent used in the mixture. The excess heat capacity presented deviations from ideality that suggest solvent-substrate interactions. Heat capacity at different temperatures can be used for thermodynamic analysis and process modeling in chemical transformations of terpenes.
Abbreviations
- C p :
-
Molar heat capacity [J mol−1⋅K−1]
- C p,i :
-
Molar heat capacity of pure compound i [J mol−1⋅K−1]
- C pM :
-
Molar heat capacity of mixture [J mol−1⋅K−1]
- \(\overline{{C }_{pM}}\) :
-
Mean value of heat capacity [J mol−1⋅K−1]
- \({C}_{pM,i}\) :
-
The experimental data [J mol−1⋅K−1]
- \({\widehat{C}}_{pM, i}\) :
-
The value predicted with the model [J mol−1·K−1]
- C E pM :
-
Excess molar heat capacity [J mol−1⋅K−1]
- k:
-
Coverage factor [-]
- n :
-
Total number of measurements [-]
- p :
-
Pressure [kPa]
- R2 :
-
Coefficient of determination [-]
- SE:
-
Standard error [J mol−1⋅K−1]
- T :
-
Temperature [K]
- u(T):
-
Standard uncertainty of temperature [K]
- x i :
-
Mole fraction of pure compound i [-]
- µDSC:
-
Micro Differential Scanning Calorimetry
References
Gilbert, L., Mercier, C.: Solvent effects in heterogeneous catalysis : application to the synthesis of fine chemicals. Stud. Surf. Sci. Catal. 78, 51–66 (1993). https://doi.org/10.1016/S0167-2991(08)63303-0
Langa, E., Palavra, A.M.F., Lourenço, M.J.V., Nieto De Castro, C.A., Mainar, A.M.: P, ρ, T and heat capacity measurements of (α-pinene + β-pinene) mixtures over the temperature range 283.15 K to 358.15 K and pressures up to 40 MPa: experiments and modelling. J. Chem. Thermodyn. 57, 493–499 (2013). https://doi.org/10.1016/j.jct.2012.09.012
Martínez-López, J.F., Schneider, S., Salavera, D., Mainar, A.M., Urieta, J.S., Pardo, J.I.: Molar heat capacities of the mixture 1,8-cineole + ethanol at several temperatures and atmospheric pressure. J. Chem. Thermodyn. 92, 146–151 (2016). https://doi.org/10.1016/j.jct.2015.09.012
Martínez-López, J.F., Pardo, J.I., Urieta, J.S., Mainar, A.M.: Isobaric molar heat capacities of the mixture (p-cymene + ethanol) at several temperatures and atmospheric pressure. J. Chem. Thermodyn. 111, 142–148 (2017). https://doi.org/10.1016/j.jct.2017.03.027
Kolicheski, M.B., Cocco, L.C., Mitchell, D.A., Kaminski, M.: Synthesis of myrcene by pyrolysis of β-pinene: analysis of decomposition reactions. J. Anal. Appl. Pyrolysis 80(1), 92–100 (2007). https://doi.org/10.1016/j.jaap.2007.01.005
Yang, G., Liu, Y., Zhou, Z., Zhang, Z.: Kinetic study of the direct hydration of turpentine. Chem. Eng. J. 168(1), 351–358 (2011). https://doi.org/10.1016/j.cej.2011.01.037
Becerra, J.A., Villa, A.L.: Thermodynamic analysis of α-pinene and limonene allylic oxidation over a FePcCl16-NH2-SiO2 Catalyst. Chem. Eng. Technol. 41(1), 124–133 (2018). https://doi.org/10.1002/ceat.201700118
Durango, E.A., A. L. V. De P, and C. M. De,: Síntesis de nopol a partir de β-pineno y aceite de trementina con el catalizador Sn-MCM-41. Rev. Fac. Ing. Univ. Antioquia 36, 44–55 (2006)
Casas-Orozco, D., Alarcón, E., Villa, A.L.: Kinetic study of the nopol synthesis by the prins reaction over tin impregnated MCM-41 catalyst with ethyl acetate as solvent. Fuel 149, 130–137 (2015). https://doi.org/10.1016/j.fuel.2014.08.067
Sánchez-Velandia, J.E., Villa, A.L.: Selective synthesis of high-added value chemicals from α-pinene epoxide and limonene epoxide isomerization over mesostructured catalysts: effect of the metal loading and solvent. Catal. Today (2021). https://doi.org/10.1016/j.cattod.2021.09.011
Fujisawa, M., Matsushita, T., Kimura, T.: Excess molar heat capacities of ((R)-(+)-α-Pinene+(S)-(-)-α-Pinene) at temperatures between 293.15-308.15 K. J. Therm. Anal. Calorim. 81, 137–139 (2005)
De Castro, C.A.N., Lourenço, M.J.V., Sampaio, M.O.: Calibration of a DSC: Its importance for the traceability and uncertainty of thermal measurements. Thermochim. Acta 347(1–2), 85–91 (2000). https://doi.org/10.1016/S0040-6031(99)00420-7
Straka, M., Růžička, K., Růžička, V.: Heat capacities of chloroanilines and chloronitrobenzenes. J. Chem. Eng. Data 52(4), 1375–1380 (2007). https://doi.org/10.1021/je700080k
Fulem, M., Laštovka, V., Straka, M., Růžička, K., Shaw, J.M.: Heat capacities of tetracene and pentacene. J. Chem. Eng. Data 53(9), 2175–2181 (2008). https://doi.org/10.1021/je800382b
Laboratories, M.P.: Thermodynamic properties of propanone ( acetone ) at temperatures from 278 K to 323 K and pressures up to 400 MPa. J. Chem. Thermodyn. 23(9), 867–876 (1991)
Low, D.I.R.: The heat capacities of acetone, methyl iodide and mixtures thereof in the liquid state. Proc. R. Soc. London. Ser. A. Math. Phys. Sci. 267(1330), 384–394 (1962). https://doi.org/10.1098/rspa.1962.0106
Yaws, C.L.: Yaws’ Handbook of Thermodynamic and Physical Properties of Chemical Compounds. KNOVEL, New york (2003)
Wagner, Z., Bendová, M., Rotrekl, J.: Thermochemical properties of selected terpenes. J. Solution Chem. 49(9–10), 1137–1153 (2020). https://doi.org/10.1007/s10953-020-01016-9
Deshpande, D.D., Patterson, D., Andreoli-Ball, L., Costas, M., Trejo, L.M.: Heat capacities, self-association and complex formation in alcohol-ester systems. J. Chem. Soc. Faraday Trans. 87(8), 1133–1139 (1991). https://doi.org/10.1039/FT9918701133
Zábranský, F., Hynek, V., Finkeová-Haštabová, J., Veselý, F.: Heat capacities of six liquid esters as a function of temperature. Chem. Phys. En Chem. Technol. Chem. 52, 251–257 (1987)
Tardajos, G., Aicart, E., Costas, M., Patterson, D.: Liquid structure and second-order mixing functions for benzene toluene and p-xylene with n-alkanes. J Chem Soc Faraday Trans. 1 Phys. Chem 82(9), 2977–2987 (1986). https://doi.org/10.1039/F19868202977
Pedersen, M.J., Kay, W.B., Hershey, H.C.: Excess enthalpies, heat capacities, and excess heat capacities as a function of temperature in liquid mixtures of ethanol + toluene, ethanol + hexamethyldisiloxane, and hexamethyldisiloxane + toluene. J. Chem. Thermodyn. 7(12), 1107–1118 (1975). https://doi.org/10.1016/0021-9614(75)90030-0
Páramo, R., Zouine, M., Casanova, C.: New batch cells adapted to measure saturated heat capacities of liquids. J. Chem. Eng. Data 47(3), 441–448 (2002). https://doi.org/10.1021/je0155103
Sampaio, M.O., Nieto De Castro, C.A.: Heat capacitiy of liquid terpenes. Fluid Phase Equilib. 150(151), 789–796 (1998). https://doi.org/10.1016/s0378-3812(98)00359-8
Cobos, J.C., Garcia, I., Casanova, C., Roux, A.H., Roux-Desgranges, G., Grolier, J.P.E.: Excess heat capacities of 1-butanol + toluene from 298 to 368 K. Fluid Phase Equilib. 69, 223–233 (1991). https://doi.org/10.1016/0378-3812(91)90035-6
Acknowledgements
N.S. Castellanos thanks to Universidad de Antioquia (UdeA) and Minciencias (previuos Colciencias) for the financial of the internship as young researcher, “Vocaciones y formación en CTel para la reactivación económica en el marco de la postpandemia 2020”, call 891. The authors thank funding from UdeA and from the Ministry of Science, Technology and Innovation, the Ministry of Education, the Ministry of Industry, Commerce and Tourism, and ICETEX, Programme Ecosistema Científico-Colombia Científica, from the Francisco José de Caldas Fund, Grant RC-FP44842-212-2018.
Funding
Open Access funding provided by Colombia Consortium. Universidad de Antioquia (UdeA) and Minciencias (previuos Colciencias), “Vocaciones y formación en CTel para la reactivación económica en el marco de la postpandemia 2020”, call 891, Ministry of Science, Technology and Innovation, the Ministry of Education,the Ministry of Industry, Commerce and Tourism, and ICETEX,Programme Ecosistema Científico-Colombia Científica, from the Francisco José de Caldas Fund, RC-FP44842-212-2018, RC-FP44842-212-2018
Author information
Authors and Affiliations
Contributions
NSC: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing—original draft. ALV: Conceptualization, Methodology, Formal analysis, Writing—review & editing, Supervision, Project administration, Funding acquisition.
Corresponding author
Ethics declarations
Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Castellanos, N.S., Villa, A.L. Heat Capacity of Various (Solvent + Terpene) Mixtures as Function of Composition and Temperature. J Solution Chem 52, 1066–1079 (2023). https://doi.org/10.1007/s10953-023-01294-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-023-01294-z