Abstract
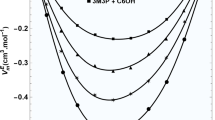
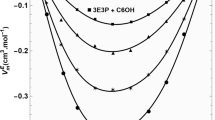
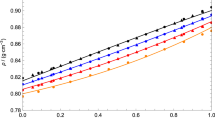
A familiar polyalcohol, 1,2-propanediol (12PDO), is among the simple alcoholic solvents with high solubility for lithium tetrafluoroborate (LiBF4), as well as a wide temperature range for liquids. The viscosity of 12PDO solutions containing LiBF4 was measured in the temperature range of 280‒340 K using a vibrational viscometer, and it exhibited non-Arrhenius behavior fitted with the Vogel–Fulcher–Tamman (VFT) equation, which is an empirical model for describing liquid dynamics. The viscosity (η) increased with the increasing mole fraction of LiBF4 (x), and the variation in η was found to be mainly controlled by the pre-exponential factor η0 in the VFT expression. The VFT-fitting parameters, the strength parameter (D), and ideal glass transition temperature (T0), which indicate the Arrhenius behavior and an ideal glass transition temperature, respectively, correlated with the thermal analysis results that were obtained via differential scanning calorimetry. With the increasing x, the value of D decreased rapidly for x < 0.20, whereas it remained approximately unchanged for x > 0.20. The concentration dependence of T0 also differed at approximately x = 0.20. The changes in D and T0 with x indicated that the intrinsic hydrogen-bonding networks between the solvent molecules were rapidly destroyed by the added ions at reduced salt concentrations, while such a structural breaking effect of the added ions weakened owing to ion associations at increased salt concentrations. Thus, it was observed that the solvation structure and dynamics of the solutions strongly depended on the salt concentration.







Similar content being viewed by others
References
Böhmer, R., Ngai, K.L., Angell, C.A., Plazek, D.J.: Nonexponential relaxations in strong and fragile glass formers. J. Chem. Phys. (1993). https://doi.org/10.1063/1.466117
Huang, D., McKenna, G.B.: New insights into the fragility dilemma in liquids. J. Chem. Phys. 114, 5621–5630 (2001)
Rosa, A.C.P., Cruz, C., Santana, W.S., Brito, E., Moret, M.A.: Non-Arrhenius behavior and fragile-to-strong transition of glass-forming liquids. Phys. Rev. E (2020). https://doi.org/10.1103/PhysRevE.101.042131
Machanová, K., Wagner, Z., Andresová, A., Rotrekl, J., Boisset, A., Jacquemin, J., Bendová, M.: Thermal properties of alkyl-triethylammonium bis{(trifluoromethyl)sulfonyl}imide ionic liquids. J. Solution Chem. (2015). https://doi.org/10.1007/s10953-015-0323-3
Takeda, K., Hirami, H., Izawa, T., Terashima, Y.: Density, viscosity, and glass transition of an ethylenediamine–ethylene glycol binary system. J. Solution Chem. (2017). https://doi.org/10.1007/s10953-017-0650-7
Wang, L.M., Tian, Y., Liu, R., Richert, R.: Calorimetric versus kinetic glass transitions in viscous monohydroxy alcohols. J. Chem. Phys. 128, 084503 (2008)
Saini, M.K., Murthy, S.S.N.: Glass formation in binary solutions of acetaminophen with guaifenesin and mephenesin. J. Solution Chem. 44, 1723–1748 (2015)
Crowley, K.J., Zografi, G.: The use of thermal methods for predicting glass-former fragility. Thermochim. Acta (2001). https://doi.org/10.1016/S0040-6031(01)00662-1
Terashima, Y., Mori, M., Sugimoto, N., Takeda, K.: Fragility and glass transition for binary mixtures of 1,2-propanediol and LiBF4. Chem. Phys. Lett. 600, 46–50 (2014)
Terashima, Y., Mori, M., Takeda, K.: Fragility and glass transition for binary mixtures of 1,2-propanediamine and NaClO4. J. Therm. Anal. Calorim. (2016). https://doi.org/10.1007/s10973-015-4781-z
Terashima, Y.: Difference in variation of glass transition activation energy between 1,2-propanediamine and 1,2-propanediol. Chem. Phys. Lett. (2016). https://doi.org/10.1016/j.cplett.2016.03.024
Terashima, Y., Takeda, K.: Effects of adding LiBF4 on the glass-transition kinetics of 1,2-propanediol. Chem. Phys. 497, 17–23 (2017)
Terashima, Y., Sugimoto, N., Mori, M., Kinoshita, N., Takeda, K.: Effects of solvents and solutes on glass transition thermodynamics and kinetic fragility for amine and alcohol solutions of inorganic salts. J. Therm. Anal. Calorim. 135, 2797–2805 (2019)
Terashima, Y., Hirai, T.: Mapping and classification of ionic liquids in terms of glass transition and fragility. J. Therm. Anal. Calorim. (2022). https://doi.org/10.1007/s10973-022-11427-z
Vogel, H.: The law of the relation between the viscosity of liquids and the temperature. Phys Z. 22, 645–646 (1921)
Fulcher, G.S.: Analysis of recent measurements of the viscosity of glasses. J. Am. Ceram. Soc. 8, 339–355 (1925)
Tammann, G., Hesse, W.: Die Abhängigkeit der Viscosität von der Temperatur bie unterkühlten Flüssigkeiten. Z. Anorg. Allg. Chem. 156, 245–257 (1926)
Wang, G., Xing, Z., Zhang, X., Liu, F., Zhang, Q.: Thermodynamic, excess properties and intermolecular interactions of ionic liquid 1- Ethyl-3-Methylimidazolium thiocyanate and propylene carbonate mixtures. J. Solution Chem. 51, 594–608 (2022)
Cai, G., Yang, S., Wang, X., Zhou, Q., Xu, J., Lu, X.: Densities and viscosities of binary mixtures containing the polyhydric protic ionic liquid(2-hydroxy-N-(2-hydroxyethyl)-N-methylethanaminium methanesulfonate) and water or alcohols. J. Solution Chem. 49, 423–457 (2020)
Wei, Y., Zhang, W., Zhang, X., Zhang, Q.: The volumetric and transport properties of 1-ethyl-3-methylimidazolium trifluoromethanesulfonate ionic liquid and propylene carbonate binary system. J. Solution Chem. (2019). https://doi.org/10.1007/s10953-019-00842-w
Satheesh, B., Sreenu, D., Jyostna, T.S.: Thermodynamic studies on non-ideal binary mixtures of isoamyl alcohol and various alkanols at 298.15 to 308.15 K. J. Solution Chem. (2021). https://doi.org/10.1007/s10953-020-01048-1
Katsu, S., Ito, S., Yoshimura, N., Takayanagi, M.: Variation in near-infrared spectra of water containing polyhydric alcohol. J. Solution Chem. (2019). https://doi.org/10.1007/s10953-019-00928-5
Doi, T., Fujii, R., Inaba, M.: Improved stability of highly concentrated LiBF4/fluorinated ethyl acetate-based electrolyte solutions with a co-solvent for LiNi0.8Co0.1Mn0.1O2 positive electrodes in lithium ion batteries. J. Appl. Electrochem. 51(2), 1535–1544 (2021)
Sashmitha, K., Rani, M.U.: A comprehensive review of polymer electrolyte for lithium-ion battery. Polym. Bull. (2022). https://doi.org/10.1007/s00289-021-04008-x
Huang, J., Li, F., Wu, M., Wang, H., Qi, S., Jiang, G., Li, X., Ma, J.: Electrolyte chemistry for lithium metal batteries. Sci. China Chem. 65, 840–857 (2022)
Viswanath, D.S., Ghosh, T.K., Prasad, D.H.L., Dutt, N.V.K., Rani, K.Y.: Viscometers. In: Viswanath, D.S., Ghosh, T.K., Prasad, D.H.L., Dutt, N.V.K., Rani, K.Y. (eds.) Viscosity of Liquids, pp. 10–107. Springer, Dordrecht, The Netherlands (2007)
Adam, G., Gibbs, J.H.: On the Temperature dependence of cooperative relaxation properties in glass-forming liquids. J. Chem. Phys. (1965). https://doi.org/10.1063/1.1696442
Wang, Y., Turk, M.C., Sankarasubramanian, M., Srivatsa, A., Roy, D., Krishnan, S.: Thermophysical and transport properties of blends of an ether-derivatized imidazolium ionic liquid and a Li+-based solvate ionic liquid. J. Mater. Sci. (2017). https://doi.org/10.1007/s10853-016-0735-5
Wu, L., Venkatanarayananan, R.I., Shi, X., Roy, D., Krishnan, S.: Glass transition, viscosity, and conductivity correlations in solutions of lithium salts in PEGylated imidazolium ionic liquids. J. Mol. Liq. (2014). https://doi.org/10.1016/j.molliq.2014.07.031
Author information
Authors and Affiliations
Contributions
The single author, YT, contributed to all processes of this study: conception and design, material preparation, data collection and analysis, and writing and revising the manuscript, and approved submitting the manuscript.
Corresponding author
Ethics declarations
Competing interests
No funds, grants, or other support was received. The author has no relevant financial or nonfinancial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Terashima, Y. Non-Arrhenius Behavior of the Viscosity and Glass Transition of 1,2-Propanediol Solutions Containing LiBF4. J Solution Chem 53, 667–679 (2024). https://doi.org/10.1007/s10953-023-01288-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-023-01288-x