Abstract
This work addresses the effect of chaotropic anions (thiocyanate and tosylate) on the solubility of glycine, L-leucine, L-phenylalanine, and L-aspartic acid in aqueous solutions at 298.2 K. The salts used were NaSCN, KSCN, NH4SCN, and NaC7H7SO3 (sodium tosylate), with salt concentrations ranging from 0 to 2 molal. The pH of the saturated solutions was registered, and solid-phase studies were also performed. All the thiocyanate salts and sodium tosylate induced a salting-in effect, except in the systems composed of glycine in aqueous sodium tosylate solutions at 0.5 and 1 molal. For L-leucine, L-phenylalanine, and L-aspartic acid the salting effect of anions followed the order tosylate− > SCN− > \({\mathrm{NO}}_{3}^{-}\) > Cl−, in good agreement with the behavior predicted by the Hofmeister series. Differently, the relative solubility of glycine in aqueous nitrate solutions was higher than in those containing thiocyanate, followed by the chloride and, closing the rank, the solutions containing the tosylate anion, suggesting that the solubility change in this case is achieved by a different mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The knowledge of amino acids (AA) solubility in aqueous salt solutions is relevant for different technical applications. For example, during the production of L-aspartic acid (L-Asp) by chemo or enzymatic synthesis, after the extraction steps, impurities, such as inorganic salts, acids, and alkalis, are still present in the system. Thus, the knowledge of the solubility of L-Asp in these aqueous salt solutions is necessary to optimize the crystallization process [1].
On the other hand, a hot topic still under debate is what may be referred to as contemporary Hofmeister solvation science [2], where ion-protein and ion-ion interactions at the protein surface and in the aqueous medium must be taken into account. An original picture establishing the line between simple chaotropes, such as thiocyanate or perchlorate, and structure-disrupting ions like sulfonates or phenolates, which act as hydrophobic/hydrotropic ions, was discussed by Leontidis [3]. More recently, using solubility, tensiometry, calorimetry data, and COSMO-RS calculations, a two-dimensional space diagram based on the nature of the anion headgroup and the hydrophobicity of the anion apolar moiety was proposed to clarify the so-called expanded Hofmeister series of ionic groups in amphiphilic molecules [4].
Contributing to clarify the ion effect on the solubility of amino acids, in our previous work [5], we demonstrated some significant inconsistencies in the published data and the absence of data for some ions such as thiocyanate. The only work found in our extensive search was the solubility of twenty amino acids in aqueous solutions of guanidinium thiocyanate at 27 °C. This salt induced an impressive solubility increase on phenylalanine, a more moderate salting-in for isoleucine, and, surprisingly, a salting-out on glycine [6].
More particularly, the study of the effect of organic-based anions is even harder to find in the open literature. Sodium p-toluenesulfonate (Na-tosylate), for instance, is a well-known hydrotrope [7]. Hydrotropes enhance the solubility of compounds poorly soluble in water, through hydrophobic interactions, between their apolar parts and those of the solute [8,9,10]. Although a large body of work has been published in the field, there is not much information on the effect of hydrotropes on the increase of zwitterions (such as amino acids) solubility in water.
To fill the gap identified for systems containing thiocyanate-based salts, in this work, the solubilities of glycine (Gly), L-leucine (L-Leu), L-phenylalanine (L-Phe), and L-aspartic acid (L-Asp) in aqueous NaSCN, KSCN, and NH4SCN solutions were measured. Besides the reasons mentioned above, the thiocyanate anion was also selected because it is located at the right end of the Hofmeister series (strong salting-in agent for proteins) and it is very interesting to discuss and compare the effect of thiocyanate anion to that of chloride, nitrate or sulphate. In addition, after investigating the impact of inorganic salts on the solubility of AA, the study was extended to the sodium salt with an organic anion, tosylate.
The chemical structures of the studied amino acids and salts are shown in Fig. 1. Leucine is a branched-chain amino acid containing an isobutyl group in the side chain, being a nonpolar aliphatic hydrophobic amino acid. Phenylalanine is also nonpolar but with an aromatic side chain, being an essential AA as leucine. The least water-soluble AA studied in this work is aspartic acid presenting two carboxylic acid groups [11]. All the measurements were performed at 298.2 K with salt molality ranging from 0 to 2 molal. The isothermal shake-flask method was combined with the gravimetric, or the refractive index methods of analysis, guaranteeing good accuracy.
2 Experimental
2.1 Chemicals
The source, CAS, and purity of the chemicals are given in Table 1. All the AAs were used without further purification and stored in a desiccator to keep them dry. The inorganic salts were dried in the oven at 343.15 K for at least 24 h and, before use, cooled in the desiccator with silica gel. The water content of the salts were checked by Karl-Fischer (KF) titration. It was found that the salts do not contain water. The electrolyte solutions were prepared using deionized water (specific resistance of 182,000 Ω·m at 298.15 K, particles with size < 0.22 μm, and total organic carbon < 5 ppb).
2.2 Solubility Experiments
The experimental setup includes the thermostatic water bath that guarantees a temperature uncertainty of 0.1 K and magnetic plates for stirring. The binary (salt and water) solutions with the molalities 0.5, 1, and 2 molal were prepared by mass (Denver Instrument, ± 0.0001 g). The saturated solution was prepared by adding a slight excess of AA into the equilibrium cell and a known amount of binary solution. After, it was placed into the water bath at 298.2 K and stirred for around 30 h to reach equilibrium. The mixing speed was between 500 and 700 rpm in all the experiments. After the stirring period, the solutions rested for at least 12 h to precipitate the undissolved AA particles. The times of stirring and rest, and the mixing speed, were studied previously and were optimized to ensure the equilibrium, meaning the changes in the measured solubility values are within the 95% confidence interval for the expected solubility value determined by selecting reliable data from the open literature.
After, four glass vessels were weighed and the samples (approximately 2–4 cm3) were collected using preheated plastic syringes (avoiding any salt or amino acid precipitation during sampling) with filters (0.45 mm pore diameter). The mass of the sample was obtained, weighing the vessels once more. Replicates were carried out when the variation coefficient of the four measured values was higher than 5%.
The amino acid content of the saturated solutions was found by the refractive index method of analysis. The refractive index was measured (at 298.2 K) in a digital refractometer (Abbemat 500, Anton Paar) with a reproducibility within ± 0.00002. The measuring range is between 1.30 and 1.72 nD. The calibration curves (R2 > 0.990), relating the amino acid concentration (in g per kg of solution) and the refractive index was built using five standard solutions of known AA composition at a fixed salt concentration. The samples were diluted with a weighed amount of (water + salt) solution to get refractive indexes within the calibration curve range. Before each set of measurements, the refractive index of pure water was checked. Finally, the refractive indexes of each solution were determined twice by placing about 1 ml of the sample on the refractometer prism. At least three independent values are used to find the final average solubility value. It is relevant to mention that the same aqueous salt solution initially prepared was used to find the calibration curve, prepare the saturated solution, and also to dilute them before the refractive index measurements. The pH of the saturated solutions was measured using a pH meter (Nahita Model 903) and pH-electrode (METTLER TOLEDO InLab Ultra-Micro-ISM). A pH calibration was carried out at 298.2 K by measuring the pH of buffer solutions.
2.3 Solid Phase Studies
The solid phase of the pure AA as received from the supplier and the solids in equilibrium with the saturated solutions, after vacuum filtration and drying at room temperature, were analyzed by powder and single-crystal X-ray diffraction.
Powder XRD data were collected on a X’Pert MPD Philips diffractometer, using Cu-Ka radiation (λ = 1.5406 Å), with a curved graphite monochromator, a set incident area of 10 mm2, and a flat plate sample holder, in a Bragg–Brentano para-focusing optics configuration. Intensity data were collected by the step counting method (step 0.02° and time 5 s) in the range 5° < 2θ < 50°.
The cell parameters of suitable crystals of L-aspartic acid, L-phenylalanine, glycine, and L-leucine before dissolution, as well the samples obtained after crystallization from the aqueous solutions of the different salts, were determined on a Bruker D8 QUEST diffractometer equipped with a Photon 100 area detector, with monochromated Mo-Kα radiation (λ = 0.71073 Å) and operating at 150(2) K. The selected crystals analyzed were put at 40 mm from the photon 100 detector and the spots were measured using different counting times (varying from 5 to 30 s).
3 Results and Discussion
3.1 Solubilities of AA in Aqueous Solutions of Salts with the Thiocyanate Anion
Table 2 reports the measured solubilities and the standard deviation (in brackets) of L-aspartic acid, L-phenylalanine, L-leucine, and glycine in the aqueous NaSCN, KSCN, and NH4SCN solutions with a salt molality of 0, 0.5, 1.0 and 2.0 at 298.2 K. In all the studied systems the absolute solubility follows Gly > Phe > Leu > Asp, which matches the solubility in pure water. The maximum coefficient of variation was 2.9% in the system water/potassium thiocyanate/L-aspartic acid at 0.5 molal salt concentration.
Figure 2 shows the relative solubilities of the studied AA in different aqueous salt solutions at 298.2 K. For glycine, L-leucine, and L-phenylalanine the solubility in pure water at 298 K was collected from the literature and is presented in Table S1. This information for L-aspartic acid was already reported in our previous work [12]. All the thiocyanate salts induced a salting-in effect over the whole salt concentration range for all the AA, matching the expected trend from the Hofmeister series and the findings by Li et al. [13] that reported by intermolecular vibrational energy exchange methods the binding of thiocyanate anions with the charged ammonium group of AA.
The magnitude of the salting-in effect of each AA is very similar in all the aqueous salt solutions with the different monovalent cations (Na+, K+, and \({\mathrm{NH}}_{4}^{+}\)), except for Leu in aqueous NH4SCN solution. In the latter system, the magnitude is higher as the relative solubility of Leu in aqueous NH4SCN solution gets very close to that of Phe, and not glycine as observed for the other salt solutions. Summarizing, the relative solubility follows the order Asp > Phe > Leu ≅ Gly in the aqueous solutions of salts with the sodium and potassium cations as in the aqueous Mg(NO3)2 and Ca(NO3)2 solutions [14]. With the ammonium cation, the ranking is Asp > Phe ≅ Leu > Gly. Even if in our previous work with the monovalent cations [5], we concluded that the monovalent cations do not interact significantly with the hydrophobic groups of the AA, the subtle differences within the different cations, in particular those observed for the ammonium, might be related to a favorable interaction of the larger ammonium cation, that presents a more disperse charge, with the apolar moieties of both Leu and Phe, in comparison to smaller sodium or potassium cations, that present a more concentrated charge.
3.2 Solubilities of AA in Aqueous Solutions of Na-Tosylate
Table 3 presents the measured solubilities and the standard deviation (in brackets) of L-aspartic acid, L-phenylalanine, L-leucine, and glycine in the aqueous Na-tosylate solutions up to 2 molal of H2O at 298.2 K. In all the studied systems, the absolute solubility follows the order Gly > Phe > Leu > Asp, which matches the solubility in pure water. The maximum coefficient of variation was 2.1% in the system water/Na-tosylate/L-aspartic acid, at 2 molal salt concentration.
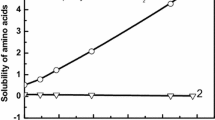
The relative solubilities of the four AA in Na-tosylate solution at 298.2 K is given in Fig. 3. Globally, Na-tosylate induces a salting-in effect for all the AA, excepting glycine, where the effect is weak. Until 1 molal, the relative solubility follows the same order as in aqueous NaSCN and KSCN solutions being L-Asp > L-Phe > L-Leu > Gly. After, the solubility of phenylalanine decreases, and the ranking changes to L-Asp > L-Leu > L-Phe > Gly. Due to this change, the solubility of phenylalanine was also studied at 1.5 molal to check the magnitude of the salting-in effect. It was observed that the relative solubility decreases only above 1.5 molal. The solid-phase analysis of the sample taken after the solubility studies in 2 molal of Na-tosylate solution showed peaks that do not belong to the original phenylalanine structure suggesting solid phase transformations. As the sodium cation does not show a strong interaction with the amino acids, it can be concluded that the tosylate anion interacts with the apolar parts of the amino acids and increases their solubilities.
To evaluate the effect of the anions on the solubility of the studied amino acids, the salts with the sodium cations were chosen. Figure 4 shows the relative solubility of glycine, L-leucine, L-phenylalanine, and L-aspartic acid in aqueous NaCl, NaNO3, NaSCN, and Na-tosylate solutions at 298.2 K. Additionally, the solubility data in aqueous Na2SO4 solution was also added for glycine, considering the consistency analysis discussed in our previous work [5]. Thus, the relative solubilities of L-leucine, L-phenylalanine, and L-aspartic acid followed the same order: tosylate > thiocyanate > nitrate > chloride, showing that the anion effect on the solubility of these amino acids is in agreement to the Hofmeister series. Even if the increase in the solubility with sodium tosylate is not significant, meaning that it does not act as an efficient hydrotrope for L-aspartic acid, due to its small apolar region and its significant polarity, it is the salt with the most relevant effect, showing the importance of the hydrophobic parts of both solute and hydrotrope, but also the much higher effect of the interaction between the apolar part of the AA with the anions.
For glycine, the tosylate anion presents a completely reversed effect, inducing a very weak salting-in and even a salting-out at 0.5 and 1 molal. The reason for this can be the smaller side chain group in glycine and the fact that glycine from the supplier used in the analysis with Na-tosylate was α-form, and not a mixture of α and γ form, as in all other systems, showing the importance of identifying the solid phase present in solubility measurements. The thiocyanate anion, which is the strongest inorganic salting-in anion in the Hofmeister series, for glycine induced a salting-in effect with a lower magnitude than the nitrate and even sulphate anions. Although the sulphate anion is a strong salting-out agent in the Hofmeister series, it induces a salting-in effect in very small amino acids like glycine. The solubility ranking observed is thus nitrate > sulphate > thiocyanate > chloride > tosylate.
No data on the effect of inorganic thiocyanate salts on the solubility of amino acids were found in the literature, with little information on the partial molar volumes changes of amino acids in the presence of NaSCN [15] or KSCN [16]. However, among the amino acids studied in this work, only limited data on glycine partial molar volume change were reported, not allowing a clear connection between both.
3.3 The pH of the Saturated Solutions
The pH of the saturated solutions was also measured at 298.2 K and are presented in Table 4. All amino acids are in the zwitterionic form (dipolar ions) in the saturated solutions, and this can be confirmed in the Chemspider plataform [17, 18].
3.4 The Solid-Phase Analysis
All the solid structures of the AAs from the supplier were analyzed by single crystal and powder diffraction and the information is presented in Table S2. The amino acids from the supplier, including the glycine (monoclinic, α-form, Fig. S1) used in the solubility experiments with Na-tosylate, show a single phase and are well characterized in the literature. The exception is glycine studied in the systems with the thiocyanate salts, which was supplied as a mixture of two phases, a monoclinic corresponding to the α-form and a hexagonal corresponding to the γ-form. The results of glycine (used for the solubility studies with the thiocyanates), L-leucine, L-phenylalanine, and L-aspartic acid from the supplier were presented in our previous work [5]. In all four aqueous solutions, the glycine solid phase (Fig. S2) is only in the hexagonal crystal system, the γ-form. Even if no data is available for thiocyanate salts, these results are consistent with previous observations [19,20,21] in which the presence of monovalent cations, independently of whether the anion is sulfate, nitrate or chloride, promote the primary nucleation of γ-glycine inhibiting the α-glycine nucleation.The crystalline forms of the other amino acids (Fig. S3, S4, and S6) in equilibrium with the saturated solutions containing the different salts did not change compared to the structures found in the solids from the suppliers. The exception is for phenylalanine in an aqueous Na-tosylate solution at 2 molal. The analysis of the crystals obtained after the crystallization showed that the peaks do not belong to phenylalanine (Fig. S5) and could be a mixture of compounds from some chemical modification of the initial material. The crystals were also obtained after the solubility studies of phenylalanine in the Na-tosylate solution with salt concentration of 1.5 molal (Fig. S4) and it was found that the solid phase did not change compared to the structure of the original solid from the supplier.
4 Conclusions
The solubilities of glycine, L-leucine, L-phenylalanine, and L-aspartic acid were studied in aqueous NaSCN, KSCN, and NH4SCN solutions at 298.2 K. The salts with the thiocyanate anion induced a salting-in effect with all the AA following the ranking Asp > Phe > Leu > Gly. The relative solubility of Gly was very close to Leu in aqueous solutions of salts with the sodium or potassium cation, while in aqueous NH4SCN solution, this similarity was found between Leu and Phe.
Furthermore, the solubilities of these four amino acids were studied in aqueous Na-tosylate solutions. Up to concentrations of 1 molal, the order observed was the same as for the thiocyanate solutions, and excepting glycine, all the AA showed a salting-in effect. Surprisingly, a solubility decrease was observed for phenylalanine at higher Na-tosylate concentrations. The analysis of the phenylalanine crystals taken after the solubility studies at 2 molal showed changes in the structure of the solid phase. Na-tosylate increased the solubility of L-leucine, L-phenylalanine, and L-aspartic acid more than any of the studied inorganic salts.
To evaluate the anion effect on the solubility of glycine, L-leucine, L-phenylalanine, and L-aspartic acid, the corresponding relative solubilities in aqueous solutions of NaCl, NaNO3, NaSCN, and Na-tosylate were compared. Excepting glycine, the relative solubility followed the order tosylate > thiocyanate > nitrate > chloride, in agreement with the Hofmeister series. For glycine, the order was nitrate > sulphate > thiocyanate > chloride > tosylate.
References
Wang, J., Wang, J., Liu, J., Wang, S., Pei, J.: Solubility of D-aspartic acid and L-aspartic acid in aqueous salt solutions from (293 to 343) K. J. Chem. Eng. Data. 55, 1735–1738 (2010). https://doi.org/10.1021/je9007102
Jungwirth, P., Cremer, P.S.: Beyond Hofmeister. Nat. Chem. 6, 261–263 (2014). https://doi.org/10.1038/nchem.1899
Leontidis, E.: Chaotropic salts interacting with soft matter: beyond the lyotropic series. Curr. Opin. Colloid Interface Sci. 23, 100–109 (2016). https://doi.org/10.1016/j.cocis.2016.06.017
Mehringer, J., Hofmann, E., Touraud, D., Koltzenburg, S., Kellermeier, M., Kunz, W.: Salting-in and salting-out effects of short amphiphilic molecules: a balance between specific ion effects and hydrophobicity. Phys. Chem. Chem. Phys. 23, 1381–1391 (2021). https://doi.org/10.1039/d0cp05491g
Aliyeva, M., Brandão, P., Gomes, J.R.B., Coutinho, J.A.P., Ferreira, O., Pinho, S.P., Ferreira, O.: Electrolyte effects on the amino acid solubility in water: solubilities of glycine, L-Leucine, L-phenylalanine, and L-aspartic acid in salt solutions of (Na+, K+, \({\mathrm{NH}}_{4}^{+}\))/(Cl−, \({\mathrm{NO}}_{3}^{-}\)). Ind. Eng. Chem. Res. 61, 5620–5631 (2022). https://doi.org/10.1021/acs.iecr.1c04562
Dooley, K.H., Castellino, F.J.: Solubility of amino acids in aqueous guanidinium thiocyanate solutions. Biochemistry 11, 1870–1874 (1972). https://doi.org/10.1021/bi00760a022
Nidhi, K., Indrajeet, S., Khushboo, M., Gauri, K., Jyoti Sen, D.: Hydrotropy: a promising tool for solubility enhancement: a review. Int. J. Drug Dev. Res. 3, 26–33 (2011). https://doi.org/10.52711/2231-5713.2022.00025
Soares, B.P., Abranches, D.O., Sintra, T.E., Leal-Duaso, A., García, J.I., Pires, E., Shimizu, S., Pinho, S.P., Coutinho, J.A.P.: Glycerol ethers as hydrotropes and their use to enhance the solubility of phenolic acids in water. ACS Sustain Chem. Eng. 8, 5742–5749 (2020). https://doi.org/10.1021/acssuschemeng.0c01032
Kumar, V.S., Raja, C., Jayakumar, C.: A review on solubility enhancement using hydrotropic phenomena. Int. J. Pharm. Pharm. Sci. 6, 1–7 (2014)
Kunz, W., Holmberg, K., Zemb, T.: Hydrotropes. Curr. Opin. Colloid Interface Sci. 22, 99–107 (2016). https://doi.org/10.1016/j.cocis.2016.03.005
Nelson, D.L., Cox, M.M.: Lehninger Principles of biochemistry. W. H. Freeman and Company, New York (2005)
Aliyeva, M., Brandão, P., Gomes, J.R.B., Coutinho, J.A.P., Held, C., Ferreira, O., Pinho, S.P.: Salt effects on the solubility of aromatic and dicarboxylic amino acids in water, submitted for publication. J. Chem. Thermodyn. (2022). https://doi.org/10.1016/j.jct.2022.106929
Li, J., Bian, H., Wen, X., Chen, H., Yuan, K., Zheng, J.: Probing ion/molecule interactions in aqueous solutions with vibrational energy transfer. J. Phys. Chem. B. 116, 12284–12294 (2012). https://doi.org/10.1021/jp306369w
Aliyeva, M., Brandão, P., Gomes, J.R.B., Coutinho, J.A.P., Ferreira, O., Pinho, S.P.: Solubilities of amino acids in aqueous solutions of chloride or nitrate salts of divalent (Mg2+ or Ca2+) cations. J. Chem. Eng. Data. 67, 1565–1572 (2022). https://doi.org/10.1021/acs.jced.2c00148
Singh, S.K., Kishore, N.: Partial molar volumes of amino acids and peptides in aqueous salt solutions at 25C and a correlation with stability of proteins in the presence of salts. J. Solution Chem. 32, 117–135 (2003). https://doi.org/10.1023/A:1022946105467
Wadi, R.K., Goyal, R.K.: Temperature dependence of apparent molar volumes and viscosity B-coefficients of amino acids in aqueous potassium thiocyanate solutions from 15 to 35C. J. Solution Chem. 21, 163–170 (1992). https://doi.org/10.1007/BF00647005
Pence, H.E., Williams, A.: Chemspider: an online chemical information resource. J. Chem. Ed. 87, 1123–1124 (2010). https://doi.org/10.1021/ed100697w
Chemspider, http://www.chemspider.com/
Yang, X., Lu, J., Wang, X.-J., Ching, C.-B.: Effect of sodium chloride on the nucleation and polymorphic transformation of glycine. J Cryst Growth. 310, 604–611 (2008). https://doi.org/10.1016/j.jcrysgro.2007.11.072
Han, G., Chow, P.S., Tan, R.B.H.: Understanding the salt-dependent outcome of glycine polymorphic nucleation. Pharmaceutics 13, 262 (2021). https://doi.org/10.3390/pharmaceutics13020262
Han, G., Chow, P.S., Tan, R.B.H.: Effects of common inorganic salts on glycine polymorphic transformation: an insight into salt-dependentpolymorphic selectivity. Cryst. Growth Des. 16, 6499–6505 (2016). https://doi.org/10.1021/acs.cgd.6b01177
Acknowledgements
This work was developed within the scope of the project CIMO-Mountain Research Center, UIDB/00690/2020 and LA/P/0007/2020 and CICECO-Aveiro Institute of Materials, UIDB/50011/ 2020, UIDP/50011/2020 and LA/P/0006/2020, financed by national funds through the Portuguese Foundation for Science and Technology (FCT)/MCTES. Mehriban Aliyeva thanks FCT and European Social Fund (ESF) for her Ph.D. grant (SFRH/BD/139355/2018).
Funding
Open access funding provided by FCT|FCCN (b-on).
Author information
Authors and Affiliations
Contributions
MA, Experiments, Data Analysis, Draft Manuscript PB, Solid Phase Studies JAPC, Funding, Reading and Writing the Manuscript OF, Experimental Methodology, Data Curation, Reading the Manuscript SPP, Funding, Reading and Writing the Manuscript, Supervision
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aliyeva, M., Brandão, P., Coutinho, J.A.P. et al. Solubilities of Amino Acids in the Presence of Chaotropic Anions. J Solution Chem 53, 527–537 (2024). https://doi.org/10.1007/s10953-023-01282-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-023-01282-3




 , glycine;
, glycine;  , L-leucine;
, L-leucine;  , L-phenylalanine, and
, L-phenylalanine, and  , L-aspartic acid in aqueous a NaSCN; b KSCN, and c NH4SCN solutions with different molalities at 298.2 K (Color figure online)
, L-aspartic acid in aqueous a NaSCN; b KSCN, and c NH4SCN solutions with different molalities at 298.2 K (Color figure online)
 , glycine;
, glycine;  , L-leucine;
, L-leucine;  , L-phenylalanine, and
, L-phenylalanine, and  , L-aspartic acid in aqueous Na-tosylate solution with different molalities at 298.2 K (Color figure online)
, L-aspartic acid in aqueous Na-tosylate solution with different molalities at 298.2 K (Color figure online)
 , NaCl;
, NaCl;  , NaNO3;
, NaNO3;  , NaSCN;
, NaSCN;  , Na2SO4 and
, Na2SO4 and  , Na-tosylate solutions with different molalities at 298.2 K
, Na-tosylate solutions with different molalities at 298.2 K