Abstract
Critically assessed data regarding Sn(IV) dioxides and hydroxy complexes have recently been challenged. Differences as large as nine orders of magnitude occur in certain of the published solubility products and other equilibrium constants, despite supposedly being derived from the same ‘reliable’ measurements. We show how these differing conclusions depend on the assignments of uncertainty in the respective experimental observations and that the divergence is due to error propagation in identifiable thermodynamic analyses. The use of Sn4+ as a ‘basis’/‘master’ species in thermodynamic modelling is deprecated. Automatic methods which enable the necessary calculations to be properly evaluated, as well as easily repeated, help uncover such mistakes. The results from the comprehensive NEA review are substantially confirmed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Serious discrepancies abound in published thermodynamic parameters for equilibria in aqueous solutions. For example, major revisions have proved necessary with reactions as basic as those for the chemical couples Fe2+/Fe0 [1] and Sn2+/Sn4+ [2] as well as for the second deprotonation of H2S [3]. Such changes can in general overturn decades of previously established equilibrium constants and other values dependent on them.
Most users of thermodynamic parameters rely on expert assessments to deal with this problem. The best sources are published by teams of international specialists working on well-circumscribed chemical systems under the auspices of organisations such as the International Union of Pure and Applied Chemistry (IUPAC) or the OECD Nuclear Energy Agency (NEA). Other (more comprehensive) compilations emanate from the highly respected US National Bureau of Standards (NBS, as it then was) and from its successor organisation, the US National Institute of Science and Technology (NIST). There are also numerous critical reviews authored by dedicated individuals or research groups. It is simple and efficient to assume such works have exhaustively covered the primary chemical literature as well as having made the most appropriate selections of values through careful and knowledgeable judgments.
However, as is frequently evident even from cursory comparisons, serious differences can arise between these various authoritative recommendations. Sometimes, it is a matter of ‘quis custodiet ipsos custodes?’ but, much more often, such changes are due to the emergence over time of new measurements, or of a perceived need for data re-interpretation. Sadly, key thermodynamic values can be based on a very limited number of experimental studies [1]. Improvements then tend to impact widely because of thermodynamic interdependencies.
Finding the best, up-to-date parameter values for thermodynamic modelling purposes can, thus be difficult, requiring expertise and considerable effort. The problem is greatly worsened by the complicated chemical relationships which can occur between chemical species in aqueous solutions. It is generally necessary to determine a large set of parameters which must be unique, thermodynamically consistent, and most reliable. This is a formidable task given that typical systems have tens or hundreds of possible chemical reactions. To tackle it, an automatic method which is general and comprehensive has accordingly been developed [4]. This computational approach has been applied in the present work to resolve recent controversial claims regarding solubility products and other equilibrium constants for Sn(IV) dioxides and hydroxy complexes [5]. The differences reported are as large as nine orders of magnitude, despite supposedly being derived from the same ‘reliable’ measured data.
2 Methodology
The JESS package of software and thermodynamic databases [4, 6,7,8] includes an established technique for achieving thermodynamic consistency between chemical reactions based on an ordered Gaussian elimination procedure [4]. The central aim is to achieve coherence between literature sources. It is important to emphasise at the outset that this technique only helps identify the particular chemical reactions which are most likely responsible for the thermodynamic inconsistency; subsequent decisions about which of the chemical reactions are preferred as more reliable depend on judgements requiring chemical expertise. However, knowing where the problem is located focusses attention on it and helps eliminate other distracting possibilities which are often multiple and complicated.
In the Gaussian elimination procedure, every reaction is described as a linear equation in which the species identities appear as the variables and the stoichiometry as the coefficients. Importantly, the reactions in this matrix are sorted so that the most reliable appears first. The Gaussian elimination, thus, determines the least-preferred species in the most-preferred reactions as those to be evaluated from each reaction’s equilibrium constant. These evaluated species are then substituted whenever they occur in the following reactions of lower priority until no further species remain to be determined. A specific example of the Bayesian-like decision-making procedure can be found in the Appendix of May and Rowland [4].
Accordingly, a set of the most reliable thermodynamically consistent reactions is produced, which taken in linear combinations establish the unique set of equilibrium constants defining the thermodynamics of the whole chemical system. Standard Gibbs energies (\(\Delta {}_{f}{G}^{0}\)) can ultimately be calculated from the equilibrium constants for the overall reactions forming each discrete species from Eq. 1
where R is the gas constant, T is temperature (K), and \(\Delta {}_{f}{G}^{0}\) is \(\Delta {G}^{0}\) for the formation reaction of the chemical species from the established set of basis species.
For instance, in a typical calculation, with the whole of the JESS chemical reaction database (called JPD), the following reactions are invoked in the formation of \( {\text{Sn}}\left( {{\text{OH}}} \right)_{4} ^{0} \). Here, the superscript zero indicates an electrically neutral species dissolved in solution.
where \({\text{Sn}}^{2 + }, \, {\text{H}}_{2} {\text{O}}, \, {\text{H}}^{ + }, \, {\text{e}}^{ - }\) are the basis species and the formation reaction is derived from the linear combination of
Since the calculation is strictly additive, the correctness of the result depends heavily on the least reliable \( {\text{log}}_{{10}}K^{0} \) in the linear combination, which here is for \( {\text{SnO}}_{2} \left( {\text{s}} \right){\mkern 1mu} + {\mkern 1mu} 2{\text{H}}_{2} {\text{O}}{\mkern 1mu} \rightleftharpoons {\text{Sn}}\left( {{\text{OH}}} \right)_{4} ^{0} \). Observe that the chemical species Sn4+ is absent in this consideration. In other words, all chemical reactions involving Sn4+ have been found inferior and hence eliminated as redundant.
The JESS process thus requires an assessment of the reliability of every equilibrium constant contained in JPD. This is done routinely by following an established strategy [4] in which numerical ‘weights’ (0–9) are assigned to each datum when it is first acquired; however, the weights can be altered readily enough afterwards, if and when it is deemed appropriate to do so. In this way, a score called the reaction’s ‘information content’ (IC) can be calculated algorithmically to represent the relative confidence which each reaction merits. ICs typically range between 0 and 999.
Obviously, the judgements made when finalising the weights are critical to the outcome. On the other hand, most of the choices are straightforward and, in practice, have little impact with well characterised reactions for which numerous equilibrium constants have been described in the chemical literature. This leaves only poorly characterised chemical reactions to be considered individually and, in particular, it directs attention to the most uncertain species in the chemical system.
3 Results
The \( {\text{log}}_{{10}}K^{0} \) values (11 in total) reported by Rai [5] were entered into JPD and processed following our usual steps, including the Gaussian elimination described above. Initially, with one exception, a reasonably high weight (5) was assigned to each value, in conformance with the source being titled a critical review. Regarding the exception, the equilibrium constant reported for reaction Na2Sn(OH)6(s) ⇌ Na2Sn(OH)6(aq) was immediately rejected because the implicit proposition that Na+ would not dissociate in solution seemed highly unlikely; since no modelling calculations are performed here, this decision has no repercussions for the present work.
The Gaussian elimination analysis showed that 6 reactions involving Sn4+ from Table 1 [5] were inconsistent with other (pre-existing) equilibrium constants in the JPD database. Most of these were linear combinations of reactions derived in part from the values presented in the NEA review for Sn [2]. This outcome was, of course, unsurprising given that Rai had himself identified major inconsistencies between the two sets of data.
Following our standard practice when inconsistencies are exposed by newly entered equilibrium constants, the 6 reactions (and their counterparts in the JPD database) were examined and modified individually, as follows.
JESS reaction 58019: Sn4+ + H2O = Sn(OH)3+ + H+
The value from Rai [5] (\( {\text{log}}_{{10}}K^{0} \) = 0.39) was preferred over all previous values, i.e. from refs [9,10,11]. This is in accord with the NEA [2] and other critical assessments [12, 13] which declined to select formation constants for the hydrolysis of Sn(IV) under acidic conditions. The implication here is that Sn(OH)3+ formation, and the associated quandaries about chloride complexation, are irrelevant for present purposes.
JESS reaction 64789: Sn4+ + 4H2O = Sn(OH) 04 + 4H+
JESS reaction 64790: Sn4+ + 5H2O = Sn(OH) −5 + 5H+
JESS reaction 64791: Sn4+ + 6H2O = Sn(OH) 2−6 + 6H+
The values from Rai [5] were rejected in preference to the relevant linear combinations of reactions with higher IC, in which the reactions Sn4+ + 2e− = Sn2+ (IC = 54) and SnO2(s) + 2H2O = Sn(OH) 40 , etc. (IC = 48) appeared most pertinent. These latter reactions were found by the JESS algorithm to be tenuous but better than the reactions shown above in bold.
JESS reaction 66851: SnO2(s) + 2H2O = Sn4+ + 4OH−
JESS reaction 80972: SnO2(am.,s) + 2H2O = Sn4+ + 4OH− where SnO2(s) is cassiterite. The values from Rai [5] were rejected in preference to the relevant linear combinations of reactions with higher IC, in which the reactions Sn4+ + 2e− = Sn2+ (IC = 54) and O2(g) + Sn(s) = SnO2(s) (IC = 62) or O2(g) + Sn(s) = SnO2(am.,s) (IC = 40) appeared most pertinent. These latter reactions were found by the JESS algorithm to be tenuous but better than the reactions shown above in bold.
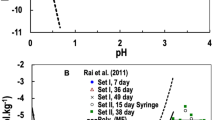
More detailed reasons supporting these actions are given under ‘Discussion’. In summary, we consider that the uncertainties proliferating in the published analyses of SnO2(s) solubility [5, 11] are worse than those from the electrochemical measurements and their extrapolation to infinite dilution by Gajda et al. [14]. Order of magnitude differences in the equilibrium constants determined by Rai [5] and Rai et al. [11] from the same solubility data illustrate the point.
Once thermodynamic consistency within JPD had thus been (re-)established, relative Gibbs energies of formation were determined in the usual way [4] for each of the Sn species in question. Equilibrium constants for the associated reactions could then be calculated straightforwardly. The results are shown in Tables 1 and 2. The general concordance (apart from Sn(OH)3+ formation) with the values from Gamsjäger et al. [2] is evident and anticipated given the decisions described above. Note that systematic errors dominate the uncertainties in these calculations so standard uncertainties would be inappropriate; the number of significant figures given here only indicates the approximate reliability of the current calculation.
Leading to the results in Table 2, linear combinations of chemical reactions (which are purely additive) give exact but not necessarily accurate values. Conversely, in modelling calculations (which are typically non-linear), uncertainties can propagate severely. Equilibrium constants determined by least-squares regression are highly prone to such numerical corruptions [15]. Much depends on the concentrations of the chemical species involved. The use of Sn4+ in alkaline solution modelling is accordingly deprecated since it can only exist at negligible concentrations under these conditions.
Despite being debatable, we do not claim here that Sn4+ is always non-existent. Unlike S2–(aq) [3], Sn4+ is plausibly conceivable. It should nevertheless be invoked with caution even in the most propitious acidic solutions. That aquated ions of such high electronic charge could have a distinguishable existence in the presence of any anion is suspect and needs to be better substantiated experimentally. In any event, for modelling purposes, chemical equilibria should never be expressed in terms of Sn4+ as a ‘basis’/‘master’ species, i.e. as a reactant. That is just troublesome and unnecessary.
4 Discussion
In a lengthy review, Rai [5] outlines numerous aspects of the chemistry applicable to this work and to the many issues presented therein which constitute serious difficulties in determining the relevant equilibrium constants to any satisfactory level of confidence. The review correctly identifies all sorts of assumptions and worryingly uncertain estimations. It specifically notes that the “main reason for the large disagreement with the values determined in this study and those reported in Gamsjäger et al. is their use of [an] extremely low \( {\text{log}}_{{10}} K_{{so}}^{0} \) resulting from the use of [a] very high (Sn4+) value” [5]. Yet, such is the complexity of Rai’s exposition even a well-informed reader might be left with doubts about the critical decisions which are essential to his argument and which underpin his conclusion. Consequently, those wishing to model the thermodynamics of Sn aqueous chemistry must, in essence, either perform the whole critical evaluation themselves or resort to making a subjective choice between the two discrepant ‘critical’ sources. These options are invidious.
Fortunately, the JESS automatic Gaussian elimination facility highlights just one critical question: how reliable is the electrochemical characterisation of Sn4+ by extrapolation to infinite dilution of the potentiometric data for Sn4+ + H2(g) ⇌ Sn2+ + 2H+ compared to that from a thermodynamic model of SnO2(s) solubility data across the pH range from 0 to 12 involving the aqueous hydroxy complexes SnOH+, \( {\text{Sn}}( {{\text{OH}}})_{4} ^{0} \), Sn(OH) –5 , and \( {\text{Sn}}( {{\text{OH}}})_{6} ^{2-} \).
Neither characterisation is ideal. The electrochemical measurements must be made in extremely concentrated acidic solutions (5–8 mol·kg–1) and, while not so extreme, the solubility measurements are also limited by electrolyte concentrations high enough to introduce significant specific effects from the (extraneous) counter-ions. Considering these uncertainties below, it is nevertheless clear that those from the former (electrochemical) determinations are the smaller (i.e. better). Other possible causes of uncertainty, detailed by Rai [5], can, thus, be set aside.
As shown in Fig. 6 of Gajda et al. [14] and reproduced as Fig. VI-2 by Gamsjäger et al. [2], the standard deviation in E0 for the reaction Sn4+ + H2(g) ⇌ Sn2+ + 2H+ is about 0.02 V, corresponding to < 4 kJ·mol–1 or < 0.7 units in a \( {\text{log}}_{{10}}K^{0} \) ~ 13. Rai dismisses these results but makes no attempt to assess their uncertainty, simply arguing (p. 1173) that “there are many reasons … to doubt the accuracy of this extrapolated value”. Emphasis on inapplicability of the SIT model to extrapolate the equilibrium constants obtained at very high ionic strengths to infinite dilution overlooks the fact that this correction is at most in the order of 0.06 V whatever interactions are occurring at high concentration. In fact, visual inspection of the data suggests a maximum error (i.e. ~ 3 SD) less than 0.03 V (which is about half of the 0.02 standard deviation estimated by Gamsjäger et al. [2]). Errors of such size cannot be responsible for \( {\text{log}}_{{10}}K^{0} \) discrepancies up to 9 orders of magnitude. Moreover, Rai’s reliance (Appendix A.3) on earlier E0s by averaging results that are grossly inconsistent (\(\Delta \) = 0.06 V) and otherwise variously problematic (as discussed by Gamsjäger et al. [2]) seems implausible.
In contrast, Rai’s recommended \( {\text{log}}_{{10}}K^{0} \) values [5] for the equilibrium constants of the aqueous hydroxy complexes \( {\text{Sn}}( {{\text{OH}}})_{4} ^{0} \), Sn(OH) –5 , and \( {\text{Sn}}({{\text{OH}}})_{6} ^{2-} \) are much more nebulous. Indeed, as Rai notes, these are essentially adjustable fitting parameters. Such sequential equilibria are always difficult to quantify, not only because they are numerically correlated but because, at higher pH, important specific interactions (e.g. with Na+) must also be taken into account. These issues are greatly exacerbated by a badly formulated framework of thermodynamic equations which, rather than using the most predominant species as it should, expresses the equilibria in terms of vanishingly small concentrations of Sn4+ (at higher pH). This is no doubt why Gamsjäger et al. avoided all reactions of hydroxy complexes formulated in terms of Sn4+ but used \( {\text{Sn}}( {{\text{OH}}})_{4} ^{0} \) instead [2, p. 114, Table VII-2]. Since the solubility of SnO2(s) is essentially invariant over 2 < pH < 8, the choice of \( {\text{Sn}}( {{\text{OH}}})_{4} ^{0} \) as a mathematical basis species reduces by two the number of adjustable modelling parameters (at higher pH). Separating the solubility data between acidic and alkaline solutions also simplifies the calculation. In particular, selected thermodynamic data for Sn4+ interactions with Cl– are relevant only in acidic solutions and so become incidental to \( {\text{Sn}}( {{\text{OH}}})_{4} ^{0} \), Sn(OH) –5 , and \( {\text{Sn}}({{\text{OH}}})_{6} ^{2-} \) formation. Thus, the solubility of crystalline and amorphous SnO2 as a function of pH is fitted equally well (or better) cf. [2, p. 116, Fig, VII-5] versus [5, Fig. 4] but modelled more robustly.
Regarding the thermodynamic constants evaluated in this work (Tables 1 and 2), significant uncertainties remain and should not be underestimated. Additional measurements are needed to improve the present situation, preferably utilising other experimental techniques. The ability to survey any new set of results and readily to explore its thermodynamic consistency with existing literature data can be a valuable tool in this endeavour. The JESS automatic facility applied herein serves that purpose.
5 Conclusion
References
Lemire, R.J., Berner, U., Musikas, C., Palmer, D.A., Taylor, P., Tochiyama, O.: OECD Chemical Thermodynamics of Iron. Part 1. Chemical Thermodynamics Series, vol. 13a. OECD Publishing, France (2013). https://doi.org/10.1787/g2g3f031-en
Gamsjäger, H., Gajda, T., Saxena, S.K., Sangster, J., Voigt, W.: Chemical Thermodynamics of Tin. OECD Chemical Thermodynamics Series, vol. 12. OECD Publishing, France (2012). https://doi.org/10.1787/9789264992061-en
May, P.M., Batka, D., Hefter, G., Königsberger, E., Rowland, D.: Goodbye to S2– in aqueous solution. Chem. Commun. (London) 54, 1980–1983 (2018). https://doi.org/10.1039/C8CC00187A
May, P.M., Rowland, D.: JESS, a Joint Expert Speciation System—VI: thermodynamically-consistent standard Gibbs energies of reaction for aqueous solutions. New J. Chem. 42, 7617–7629 (2018). https://doi.org/10.1039/C7NJ03597G
Rai, D.: Thermodynamic data for Sn(IV) dioxides and hydroxido complexes: a critical review. J. Solution Chem. 51, 1169–1186 (2022). https://doi.org/10.1007/s10953-022-01188-6
May, P.M., Murray, K.: JESS, a Joint Expert Speciation System—I. Raison d’être. Talanta 38, 1409–1417 (1991). https://doi.org/10.1016/0039-9140(91)80289-C
May, P.M., Murray, K.: JESS, a Joint Expert Speciation System - II The thermodynamic database. Talanta 38, 1419–1426 (1991). https://doi.org/10.1016/0039-9140(91)80290-G
May, P.M., Rowland, D., Königsberger, E., Hefter, G.: JESS, a Joint Expert Speciation System—IV: A large database of aqueous solution physicochemical properties with an automatic means of achieving thermodynamic consistency. Talanta 81, 142–148 (2010). https://doi.org/10.1016/j.talanta.2009.11.049
Högfeldt, E.: IUPAC Chemical Data Series No. 21. Stability Constants of Metal-ion Complexes, 2nd Suppl., Part A, Inorganic Ligands. Pergamon, Oxford (1982)
Brown, P.L., Wanner, H.: Predicted Formation Constants Using the Unified Theory of Metal Ion Complexation. Organisation for Economic Co-operation and Development. Nuclear Energy Agency, Paris (1987)
Rai, D., Yui, M., Schaef, T., Kitamura, A.: Thermodynamic model for SnO2(cr) and SnO2(am) solubility in the aqueous Na+-H+-OH–-Cl–-H2O system. J. Solution Chem. 40, 1155–1172 (2011). https://doi.org/10.1007/s10953-011-9723-1
Lothenbach, B., Ochs, M., Wanner, H., Yui, M.: Report TN8400 99–011. Thermodynamic Data for the Speciation and Solubility of Pd, Pb, Sn, Sb, Nb, and Bi in Aqueous Solution. Admin. Div. Tokai Works, Jap. Nucl. Cycle Develop. Inst., Japan (1999).
Hummel, W., Berner, U., Curti, E., Pearson, F.J., Thoenen, T.: NAGRA/PSI chemical thermodynamic database 01/01. Radiochim. Acta 90, 805–813 (2002). https://doi.org/10.1524/ract.2002.90.9-11_2002.805
Gajda, T., Sipos, P., Gamsjäger, H.: The standard electrode potential of the Sn4+/Sn2+ couple revisited. Monatsh. Chem. 140, 1293–1303 (2009). https://doi.org/10.1007/s00706-009-0188-5
May, P.M., Rowland, D.: Thermodynamic modeling of aqueous electrolyte systems: current status. J. Chem. Eng. Data 62, 2481–2495 (2017). https://doi.org/10.1021/acs.jced.6b01055
Acknowledgements
Prof. Eric F. May (University of Western Australia) is thanked for helpful suggestions.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
This work was jointly conceptualised. PMM wrote the first draft. Both authors reviewed the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
May, P.M., Filella, M. Thermodynamic Data for Sn(IV) Species in Aqueous Solution: A Matter of Controversy and Error. J Solution Chem 52, 754–761 (2023). https://doi.org/10.1007/s10953-023-01259-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-023-01259-2