Abstract
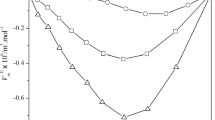
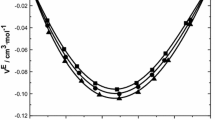
The densities, \(\rho\) and speeds of sound, u were measured for pure and the binary mixtures of monoethanolamine (MEA) + 1-alcohols (1-propanol, 1-butanol, 1-pentanol and 1-hexanol) at (298.15–318.15) K and the whole composition range. From the experimental data excess molar volumes, \({V}_{\text{m}}^{\text{E}}\), excess partial molar volumes, \({\overline{V}}_{i}^{\text{E}},\) excess thermal expansion coefficients, \({\alpha }^{\text{E}}\), isothermal coefficients of excess molar enthalpies, \((\partial {H}_{\text{m}}^{\text{E}}/\partial P)_{T,x}\), isentropic compressibility, \({k}_{\text{s}}^{ }\) and excess isentropic compressibilities, \({k}_{\text{s}}^{\text{E}}\) have been calculated for the binary systems. The excess molar volume and excess isentropic compressibility were correlated with the Redlich–Kister polynomial equation. The excess molar volumes and excess isentropic compressibilities were negative for all binary systems, except, for the system of MEA + 1-hexanol in which excess molar volume showed a sigmoid shape. The effect of temperature and chain length of alcohol on the excess molar volumes, \({V}_{\text{m}}^{\text{E}}\), and excess isentropic compressibilities, \({k}_{\text{s}}^{\text{E}}\), are discussed in terms of molecular interactions between unlike molecules. The Extended Real Associated Solution model was applied to correlate the excess molar volumes.





Similar content being viewed by others
References
Li, Z., Zhao, D., Zhuang, Y., Yang, F., Liu, X., Chen, Y.: Volumetric properties of monoethanolamine and alcohol binary mixtures at different temperatures and 0.1 MPa. J. Chem. Thermodyn. 133, 37–45 (2019)
Dean, R., Moulins, J., MacInnis, A., Palepu, R.M.: Excess volumes, partial molar and adiabatic compressibilities of binary mixtures of n-alcohols with monoethanolamine. Phys. Chem. Liq. 47, 302–310 (2009)
Lee, M.-J., Lin, T.-K., Pai, Y.-H., Lin, K.-S.: Density and viscosity for monoethanolamine + 1-propanol, + 1-hexanol, and + 1-octanol. J. Chem. Eng. Data 42, 854–857 (1997)
Kermanpour, F., Kheyrabadi, Z.G.: Experimental study of some thermodynamic properties of binary mixtures containing 3-amino-1-propanol, 2-aminoethanol, and 1-butanol at temperatures of 293.15–333.15 K to model the excess molar volumes using the PFP theory. J. Chem. Eng. Data 65, 5360–5368 (2020)
Páramo, R., Alonso, V., González, J.A., de la Fuente, I.G., Casanova, C., Cobos, J.C.: Thermodynamics of mixtures containing amines xiv. cpme of benzylamine with heptane at 293.15 K or with methanol, 1-propanol or 1-pentanol at 293.15–308.15 K. Thermochim. Acta 586, 75–79 (2014)
Segade, L., Jiménez de Llano, J., Domínguez-Pérez, M., Cabeza, Ó., Cabanas, M., Jiménez, E.: Density, surface tension, and refractive index of octane + 1-alkanol mixtures at T = 298.15 K. J. Chem. Eng. Data 48, 1251–1255 (2003)
Riddick, J.A., Bunger, W.B., Sakano, T.K.: Organic Solvents: Physical Properties and Methods of Purification. Wiley, New York (1986)
Langa, E., Mainar, A.M., Pardo, J.I., Urieta, J.S.: Excess enthalpy, excess volume, and speed of sound deviation for the mixtures β-pinene+ ethanol and β-pinene + 1-propanol at (283.15, 298.15, and 313.15) K. J. Chem. Eng. Data 50, 1255–1261 (2005)
Du, W., Wang, X.: Density and viscosity for binary mixtures of methyl decanoate with 1-propanol, 1-butanol, and 1-pentanol. J. Mol. Liq. 294, 111647 (2019)
Fatima, U., Anwar, N., Montes-Campos, H., Varela, L.M.: Molecular dynamic simulation, molecular interactions and structural properties of 1-butyl-3-methylimidazolium bis (trifluoromethylsulfonyl) imide + 1-butanol/1-propanol mixtures at (298.15–323.15) K and 0.1 M Pa. Fluid Phase Equilib. 472, 9–21 (2018)
Ramos-Estrada, M., López-Cortés, I.Y., Iglesias-Silva, G.A., Pérez-Villaseñor, F.: Density, viscosity, and speed of sound of pure and binary mixtures of ionic liquids based on sulfonium and imidazolium cations and bis (trifluoromethylsulfonyl) imide anion with 1-propanol. J. Chem. Eng. Data 63, 4425–4444 (2018)
Makhlouf, H., Muñoz-Rujas, N., Aguilar, F., Belhachemi, B., Montero, E.A., Bahadur, I., Negadi, L.: Density, speed of sound and refractive index of mixtures containing 2-phenoxyethanol with propanol or butanol at various temperatures. J. Chem. Thermodyn. 128, 394–405 (2019)
Vercher, E., Orchilles, A.V., Miguel, P.J., Martínez-Andreu, A.: Volumetric and ultrasonic studies of 1-ethyl-3-methylimidazolium trifluoromethanesulfonate ionic liquid with methanol, ethanol, 1-propanol, and water at several temperatures. J. Chem. Eng. Data 52, 1468–1482 (2007)
Pena, M.D., Tardajos, G.: Isothermal compressibilities of n-alcohols from methanol to 1-dodecanol at 298.15, 308.15, 318.15, and 333.15 K. J. Chem. Thermodyn. 11, 441–445 (1979)
Domańska, U., Pobudkowska, A., Wiśniewska, A.: Solubility and excess molar properties of 1, 3-dimethylimidazolium methylsulfate, or 1-butyl-3-methylimidazolium methylsulfate, or 1-butyl-3-methylimidazolium octylsulfate ionic liquids with n-alkanes and alcohols: analysis in terms of the PFP and FBT models. J. Solution Chem. 35, 311–334 (2006)
Chandraiah, T., Karlapudi, S., Govinda, V., Sreedhar, N., Bahadur, I.: Effect of alkyl group of 1-alkanol on molecular interactions of ethanoic acid mixtures: FT-IR spectroscopic and volumetric studies. J. Mol. Liq. 255, 354–363 (2018)
Zorębski, E., Chorążewski, M., Tkaczyk, M.: Excess molar heat capacities for (1-butanol + 1,3-butanediol) at temperatures from (285 to 353) K. J. Chem. Thermodyn. 37, 281–287 (2005)
Counsell, J., Lees, E., Martin, J.: Thermodynamic properties of organic oxygen compounds. Part XIX. Low-temperature heat capacity and entropy of propan-1-ol, 2-methylpropan-1-ol, and pentan-1-ol. J. Chem. Soc. A. (1968). https://doi.org/10.1039/J19680001819
Živković NV, Majstorović DM, Kijevčanin ML, Živković EM: Volumetric and viscometric study of 1-hexanol-based binary systems: experimental determination and modeling. J. Chem. Eng. Data 65, 3044 (2020)
Vargas-Ibáñez, L.T., Cano-Gómez, J.J., Iglesias-Silva, G.A., Santos-López, I.A., Benavides-Moran, O.E., Zwolinski, P.: Physical properties of biodiesel blended with hexanol isomers at different temperatures: surface tension, density, viscosity, and refractive index. J. Chem. Eng. Data 65, 3044–3062 (2020)
Mirheydari, S.N., Barzegar-Jalali, M., Faraji, S., Shekaari, H., Martinez, F., Jouyban, A.: Volumetric and acoustic properties of ionic liquid, 1-hexyl-3-methylimidazolium bromide in 1-hexanol, 1-heptanol and 1-octanol at T = (298.15–328.15) K. Phys. Chem. Liq. 58, 545–558 (2020)
van Miltenburg, J.C., Gabrielová, H., Růžička, K.: Heat capacities and derived thermodynamic functions of 1-hexanol, 1-heptanol, 1-octanol, and 1-decanol between 5 K and 390 K. J. Chem. Eng. Data 48, 1323–1331 (2003)
Álvarez, E., Cerdeira, F., Gómez-Diaz, D., Navaza, J.M.: Density, speed of sound, isentropic compressibility, and excess volume of (monoethanolamine + 2-amino-2-methyl-1-propanol), (monoethanolamine + triethanolamine), and (monoethanolamine + N-methyldiethanolamine) at temperatures from (293.15 to 323.15) K. J. Chem. Eng. Data 55, 994–999 (2010)
Hawrylak, B., Burke, S.E., Palepu, R.: Partial Molar and Excess volumes and adiabatic compressibilities of binary mixtures of ethanolamines with water. J. Solution Chem. 29, 575–594 (2000)
Chiu, L.-F., Liu, H.-F., Li, M.-H.: Heat capacity of alkanolamines by differential scanning calorimetry. J. Chem. Eng. Data 44, 631–636 (1999)
Zarei, H.A., Jalili, F.: Densities and derived thermodynamic properties of (2-methoxyethanol+ 1-propanol, or 2-propanol, or 1, 2-propandiol) at temperatures from T = (293.15 to 343.15) K. J. Chem. Thermodyn. 39, 55–66 (2007)
Heydarian, S., Almasi, M., Saadati, Z.: Thermophysical study of binary mixtures of 1-butyl-3-methylimidazolium nitrate ionic liquid+ alcohols at different temperatures. J. Chem. Thermodyn. 135, 345–351 (2019)
Iloukhani, H., Khanlarzadeh, K.: Volumetric properties for binary and ternary systems consist of 1-chlorobutane, n-butylamine and isobutanol at 298.15 K with application of the Prigogine–Flory–Patterson theory and ERAS-model. Thermochim. Acta 502, 77–84 (2010)
Gao, H., Yu, Z., Wang, H.: Densities and volumetric properties of binary mixtures of amino acid ionic liquid [bmim][Glu] or [bmim][Gly] with benzylalcohol at T = (298.15 to 313.15) K. J. Chem. Thermodyn. 42, 640–645 (2010)
Flory, P.D.: Statistical thermodynamics of liquid mixtures. J. Am. Chem. Soc. 87, 1833–1838 (1965)
Kretschmer, C.B., Wiebe, R.: Thermodynamics of alcohol-hydrocarbon mixtures. J. Chem. Phys. 22, 1697–1701 (1954)
Radović, I., Grozdanić, N., Djordjević, B., Šerbanović, S., Kijevčanin, M.: Prediction of excess molar volumes of binary mixtures by Prigogine–Flory–Patterson (PFP) and Extended Real Association Solution (ERAS) models. J. Serb. Chem. Soc. 82, 1379–1393 (2017)
Khanlarzadeh, K., Iloukhani, H.: Application of ERAS-model and Prigogine–Flory–Patterson theory to excess molar volumes for ternary mixtures of (2-chlorobutane+ butylacetate+ isobutanol) at T = 298.15 K. J. Chem. Thermodyn. 43, 1583–1590 (2011)
Heintz, A., Papaioannou, D.: Excess enthalpies of alcohol + amine mixtures. Experimental results and theoretical description using the ERAS-model. Thermochim. Acta 310, 69–76 (1998)
Oswal, S.: Studies of viscosity and excess molar volume of binary mixtures: 5. Characterization of excess molar volume of 1-alkanol with alkylamines, dialkylamines and trialkylamines in terms of the ERAS Model. Thermochim. Acta 425, 59–68 (2005)
Redlich, O., Kister, A.: Algebraic representation of thermodynamic properties and the classification of solutions. Ind. Eng. Chem. 40, 345–348 (1948)
Funke, H., Wetzel, W., Heintz, A.: New applications of the ERAS model. Thermodynamics of amine + alkane and alcohol + amine mixtures. Pure Appl. Chem. 61, 1429–1439 (1989)
Domańska, U., Laskowska, M.: Phase equilibria and volumetric properties of (1-ethyl-3-methylimidazolium ethylsulfate + alcohol or water) binary systems. J. Solution Chem. 37, 1271–1287 (2008)
González, J.A., de la Fuentá, I.G., Cobos, J.C.: Thermodynamics of mixtures with strongly negative deviations from Raoult’s law: Part 4. Application of the DISQUAC model to mixtures of 1-alkanols with primary or secondary linear amines. Comparison with dortmund UNIFAC and ERAS Results. Fluid Phase Equilib. 168, 31–58 (2000)
Zaichikov, A., Krestyaninov, M., Antonova, O.: Thermodynamic characteristics of self-associated aminoalcohols. J. Therm. Anal. Calorim. 115, 1857–1861 (2014)
Shirazi, S.G., Kermanpour, F.: Density and viscosity of 2-butanol + (1-propanol, 2-propanol, or 3-amino-1-propanol) mixtures at temperatures of (293.15 to 323.15) K: application of the ERAS model. J. Chem. Eng. Data 64, 2292–2302 (2019)
Villa, S., Riesco, N., de la Fuente, I.G.A., González, J., Cobos, J.: Thermodynamics of mixtures with strongly negative deviations from Raoult’s law. Part 8. Excess molar volumes at 298.15 K for 1-alkanol + isomeric amine (C6H15N) systems: characterization in terms of the ERAS model. Fluid Phase Equilib. 216, 123–133 (2004)
Benson, G.C., Kiyohara, O.: Evaluation of excess isentropic compressibilities and isochoric heat capacities. J. Chem. Thermodyn. 11, 1061–1064 (1979)
Acknowledgements
We are thankful to Bu-Ali Sina University for providing the necessary facilities to carry out the work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Forghani, F., Iloukhani, H. & Khanlarzadeh, K. Volumetric and Acoustic Investigation on the Binary Mixtures of Monoethanolamine + 1-Alcohols (C3–C6) at Different Temperatures from Experimental and Theoretical Points of View. J Solution Chem 52, 385–412 (2023). https://doi.org/10.1007/s10953-022-01236-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-022-01236-1