Abstract
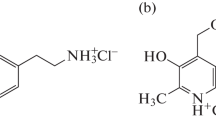
Cyclic voltammetry (CV), 1H nuclear magnetic resonance (1H NMR), density functional theory (DFT), quantum theory of atoms in molecules (QTAIM) and reduced density gradient (RDG) were used to study the interactions of dopamine hydrochloride (DH) with nicotinic acid (NAC) in aqueous solution. The results revealed that there existed three weak interactions in the DH–NAC system, C–H···π, π···π stacking and hydrogen bonds. These interactions showed different effects on the DH electrooxidation and its chemical environment. Moreover, the CV and 1H NMR results indicated that C–H···π interaction between the H atoms on the benzene ring of DH and the pyridine ring of NAC, and π···π stacking between the benzene ring of DH and the pyridine ring of NAC are the main interactions in the system.






Similar content being viewed by others
References
Broome, S.T., Louangaphay, K., Keay, K.A., Leggio, G.M., Musumeci, G., Castorina, A.: Dopamine: an immune transmitter. Neural. Regen. Res. 15(12), 2173–2185 (2020)
Bucolo, C., Leggio, G.M., Drago, F., Salomone, S.: Dopamine outside the brain: the eye, cardiovascular system and endocrine pancreas. Pharmacol. Therapeut. 203, 107392 (2019)
Klein, M.O., Battagello, D.S., Cardoso, A.R., Hauser, D.N., Bittencourt, J.C., Correa, R.G.: Dopamine: functions, signaling, and association with neurological disease. Cell. Mol. Neurobiol. 39(1), 31–59 (2019)
Li, Y.Y., Chiu, C.C., Wang, J.J., Chen, Y.W., Hung, C.H.: Dopamine enhancement of dextrorphan-induced skin antinociception in response to needle pinpricks in rats. Pharmacol. Rep. 71(4), 732–737 (2019)
Li, J., Duan, H.Y., Wei, W.Z., Luo, S.L.: Spectrometric investigations on the binding of dopamine to bovine serum albumin. Phys. Chem. Liq. 50(4), 453–464 (2012)
Djemil, R., Khatmi, D.: Quantum mechanical study of complexation of dopamine and epinephrine with β-cyclodextrin using PM6, ONIOM and NBO analysis. J. Comput. Theor. Nanosci. 9(10), 1571–1576 (2012)
Liu, M.M., Han, S.M., Zheng, X.W., Han, L.L., Liu, T., Yu, Z.Y.: Experimental and theoretical prediction of the redox potential of dopamine and its supramolecular complex with glycine. Int. J. Electrochem. Sci. 10, 235–247 (2015)
Zhai, C.P., Peng, P., Liu, X.J., Chen, X., Li, L.N.: Experimental and theoretical study on the interactions between dopamine hydrochloride and nicotinamide. J. Mol. Struct. 1178, 599–605 (2019)
Zhai, C.P., Sun, F., Zhang, P., Ma, H.T., Song, A.X., Hao, J.C.: Interactions of dopamine and dopamine hydrochloride with ethanol. J. Mol. Liq. 223, 420–426 (2016)
Zhang, P., Peng, P., Hou, B.B., Zhai, C.P.: Hydrogen bond interactions of dopamine hydrochloride with urea. Phys. Chem. Liq. 57(6), 746–754 (2019)
Zhai, C.P., Ma, H.T., Sun, F., Li, L.N., Song, A.X.: Experimental and theoretical study on the interaction of dopamine hydrochloride with H2O. J. Mol. Liq. 215, 481–485 (2016)
Hegyi, J., Schwartz, R.A., Hegyi, V.: Pellagra: dermatitis, dementia, and diarrhea. Int. J. Dermatol. 43(1), 1–5 (2004)
Gonçalves, E.M., Joseph, A., Conceição, A.C.L., da Piedade, M.E.M.: Potentiometric titration study of the temperature and ionic strength dependence of the acidity constants of nicotinic acid (niacin). J. Chem. Eng. Data 56(6), 2964–2970 (2011)
Hellenbrand, W., Boeing, H., Robra, B.-P., Seidler, A., Vieregge, P., Nischan, P., Joerg, J., Oertel, W.H., Schneider, E., Ulm, G.: Diet and Parkinson’s disease II: a possible role for the past intake of specific nutrients. results from a self-administered food-frequency questionnaire in a case-control study. Neurology 47(3), 644–650 (1996)
Fall, P.-A., Fredrikson, M., Axelson, O., Granérus, A.-K.: Nutritional and occupational factors influencing the risk of Parkinson’s disease: a case-control study in Southeastern Sweden. Movement Disord. 14(1), 28–37 (1999)
Abdullah, K.M., Alam, M.M., Iqbal, Z., Naseem, I.: Therapeutic effect of vitamin B3 on hyperglycemia, oxidative stress and DNA damage in alloxan induced diabetic rat model. Biomed. Pharmacother 105, 1223–1231 (2018)
Tyunina, E.Y., Badelin, V.G., Mezhevoi, I.N.: Study on the interaction of nicotinic acid with L-phenylalanine in buffer solution by heat capacity measurements at various temperatures. J. Solution Chem. 46(2), 249–258 (2017)
Terekhova, I.V., De Lisi, R., Lazzara, G., Milioto, S., Muratore, N.: Volume and heat capacity studies to evidence interactions between cyclodextrins and nicotinic acid in water. J. Therm. Anal. Calorim. 92(1), 285–290 (2008)
Badelin, V.G., Tyunina, E.Y., Nezhevoi, I.N., Tarasova, G.N.: Thermodynamic characteristics of molecular interactions between L-tryptophan and nicotinic acid and uracyl in aqueous buffer solutions at 298 K. Russ. J. Phys. Chem. A 89(12), 2229–2233 (2015)
Nakashima, Y., Sanada, H., Utsuki, Y., Kawada, S.: Effect of nicotinic acid on catecholamine synthesis in rat brain. J. Nutr. Sci. Vitaminol. 24(2), 67–76 (1978)
Kim, B., Kim, J.E., Lee, S.M., Lee, S.H., Lee, J.W., Kim, M.K., Lee, K.J., Kim, H., Lee, J.D., Choi, K.Y.: N-nicotinoyl dopamine, a novel niacinamide derivative, retains high antioxidant activity and inhibits skin pigmentation. Exp. Dermatol. 20(11), 943–958 (2011)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H.P., Izmaylov, A.F., Bloino, J., Zheng, G., Sonnenberg, J.L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery, J.A., Peralta, J.E., Ogliaro, F., Bearpark, M., Heyd, J.J., Brothers, E., Kudin, K.N., Staroverov, V.N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., rega, N., Millam, J.M., Klene, M., Knox, J.E., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Martin, E.L., Morokuma, K., Zakrzewski, V.G., Voth, G.A., Salvador, P., Dannenberg, J.J., Dapprich, S., Daniels, A.D., Farkas, O., Foresman, J.B., Ortiz, J.V., Cioslowski, J., Fox, D.J.: Gaussian 09, Revision D. 01, Gaussian Inc., Wallingford (2009)
Chacón, K.N., Espinal, J.F., Montero-Campillo, M.M., Yáñez, M., Mejía, S.M.: Looking for the azeotrope: a computational study of (ethanol)6–water, (methanol)6–water, (ethanol)7, and (methanol)7 heptamers. J. Phys. Chem. A 124, 7080–7087 (2020)
Su, T.Z., Tang, Z., Yin, C., Yang, Y., Wang, H.T., Peng, L., Su, Y.Z., Su, P.F., Li, J.: Insights into quaternary ammonium-based ionic liquids series with tetrafluoroborate anion for CO2 capture. J. Mol. Liq. 327, 114857 (2021)
Boys, S.F., Bernardi, F.: The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 19(4), 553–566 (1970)
Lu, T., Chen, F.W.: Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33(5), 580–592 (2012)
Humphrey, W., Dalke, A., Schulten, K.: VMD: visual molecular dynamics. J. Mol. Graphics 14(1), 33–38 (1996)
Sun, Y.L., Chen, M.F., Chen, L.G., Wang, X.L., Wang, T.: Electrochemical behaviors of ceftazidime at Gr/GCE and its interaction with DNA studied by fluorescence and CV method. Int. J. Electrochem. Sci. 14, 6522–6531 (2019)
Bagnoa, A., Rastrellia, F., Saiellib, G.: NMR techniques for the investigation of solvation phenomena and non-covalent interactions. Prog. Nucl. Mag. Resonan. Spectrosc. 47, 41–93 (2005)
Nishio, M.: The CH/π hydrogen bond in chemistry. Conformation, supramolecules, optical resolution and interactions involving carbohydrates. Phys. Chem. Chem. Phys. 13, 13873–13900 (2011)
Cheng, H., Qin, C., Yang, B., Hu, X.J., Waigi, M.G., Vasilyeva, G.K., Gao, Y.Z.: Non-covalent binding interaction between phthalic acid esters and DNA. Environ. Int. 161, 107095 (2022)
Curtis, M.D., Cao, J., Kampf, J.W.: Solid-state packing of conjugated oligomers: from π-stacks to the herringbone structure. J. Am. Chem. Soc. 126(13), 4318–4328 (2004)
Jennings, W.B., Farrell, B.M., Malone, J.F.: Stereodynamics and edge-to-face CH-π aromatic interactions in O-phenethyl-substituted biaryls. J. Org. Chem. 71(6), 2277–2282 (2006)
Malathy Sony, S.M., Ponnuswamy, M.N.: Nature of π-interactions in nitrogen-containing heterocyclic systems: a structural database analysis. Cryst. Growth Des. 6(3), 736–742 (2006)
Mohan, N., Vijayalakshmi, K.P., Koga, N., Suresh, C.H.: Comparison of aromatic NH···π, OH···π, and CH···π interactions of alanine using MP2, CCSD, and DFT methods. J. Comput. Chem. 31(16), 2874–2882 (2010)
Bondi, A.: Van der Waals volumes and radii. J. Phys. Chem. 68(3), 441–451 (1964)
Wang, H.K., Huang, Z.G., Shen, T.T., Guo, L.F.: Hydrogen-bonding interactions in adrenaline-water complexes: DFT and QTAIM studies of structures, properties, and topologies. J. Mol. Model. 18(7), 3113–3123 (2012)
Huang, Z.G., Dai, Y.M., Yu, L.: Density functional theory and topological analysis on the hydrogen bonding interactions in N-protonated adrenaline-DMSO complexes. Struct. Chem. 21(4), 863–872 (2010)
Kirn, C.K., Zhang, H., Yoon, S.H., Won, J., Lee, M.J., Kim, C.K.: Effects of basis set superposition error on optimized geometries and complexation energies of organo-alkali metal cation complexes. J. Phys. Chem. A 113(2), 513–519 (2009)
Quiñonero, D., Frontera, A., Garau, C., Ballester, P., Costa, A., Deyà, P.M.: Interplay between cation-π, anion-π and π-π interaction. ChemPhysChem 7(12), 2487–2491 (2006)
Gil, A., Branchadell, V., Calhorda, M.J.: A theoretical study of methylation and CH/π interactions in DNA intercalation: methylated 1,10-phenanthroline in adenine-thymine base pairs. RSC Adv. 6(89), 85891–85902 (2016)
Nakanishi, W., Hayashi, S., Narahara, K.: Atoms-in-molecules dual parameter analysis of weak to strong interactions: behaviors of electronic energy densities versus laplacian of electron densities at bond critical points. J. Phys. Chem. A 112(51), 13593–13599 (2008)
Koch, U., Popelier, P.L.A.: Characterization of C-H-O hydrogen bonds on the basis of the charge density. J. Phys. Chem. 99(24), 9747–9754 (1995)
Parthasarathi, R., Subramanian, V., Sathyamurthy, N.: Hydrogen bonding without borders: an atoms-in-molecules perspective. J. Phys. Chem. A 110(10), 3349–3351 (2006)
Kazachenko, A.S., Akman, F., Abdelmoulahi, H., Issaoui, N., Malyar, Y.N., Al-Dossary, O., Wojcik, M.J.: Intermolecular hydrogen bonds interactions in water clusters of ammonium sulfamate: FTIR, X-ray diffraction, AIM, DFT, RDG, ELF. NBO analysis. J. Mol. Liq. 342, 117475 (2021)
Johnson, E.R., Keinan, S., Mori-Sánchez, P., Contreras-García, J., Cohen, A.J., Yang, W.T.: Revealing noncovalent interactions. J. Am. Chem. Soc. 132(18), 6498–6506 (2010)
Zhang, Y.N., Li, J.W., Yin, Z.P., Zhang, J., Guo, W.Y., Wang, M.H.: Quantum chemical study of the carbon dioxide-philicity of surfactants: effects of tail functionalization. Langmuir 36, 15352–15361 (2020)
Acknowledgements
This work was supported by the Teacher Education Curriculum Reform Research Project of Henan Province (2020-JSJYZD-019) and the National Natural Science Foundation of China (21603059).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared that they have no conflicts of interest to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, M., Wu, Y., Li, T. et al. Experimental and Theoretical Studies on the Interaction of Dopamine Hydrochloride with Nicotinic Acid. J Solution Chem 51, 1508–1521 (2022). https://doi.org/10.1007/s10953-022-01206-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-022-01206-7