Abstract
Tin is an important element in the nuclear fuel cycle. The two latest studies/reviews (Gamsjäger et al. in Chemical Thermodynamics of Tin. Chemical Thermodynamics, vol. 12. OECD, Nuclear Energy Agency Data Bank, 2012; Rai et al. in J Solution Chem 40:1155–1172, 2011) for solubility products of Sn(IV) dioxides and Sn(IV)-hydroxido complexes were published before 2013. Since then, no significant new data have become available. Although both of these studies used some of the same experimental data, the reported values for the solubility products and the Sn(IV)-hydroxido complexes differ by about 7–9 orders of magnitude. Critical re-analyses of the key available reliable data show that the observed differences in the previously reported values are due to an extremely low solubility product value (log10 \({K}_{\text{so}}^{0}\) = − 71.60 ± 0.68) for cassiterite that resulted from a very high estimated Δf \({G}_{\text{m}}^{0}\)(Sn4+) (Gamsjäger et al. 2012). The possible underlying reasons for the estimated high Δf \({G}_{\text{m}}^{0}\)(Sn4+) are addressed. Thermodynamic data developed in this study are consistent with all of the available reliable experimental data for Sn(IV) as well as relevant data for other tetravalent elements. The new values, which differ by about 0.82 log10 units from those reported in Rai et al. (2011) and approximately 9 log10 units from Gamsjäger et al. (2012), provide predicted Sn concentrations in equilibrium with SnO2(cass) and SnO2(am) that are in close agreement with observed concentrations. The recommended log10 K0 values based on this review are − 63.57 ± 0.30 for the reaction (SnO2(s) + 2H2O ⇌ Sn4+ + 4OH−) for SnO2(cass) and − 60.98 ± 0.29 for SnO2(am), and 0.39 ± 0.1, ≤ − 2.36, − 10.02 ± 0.34, − 21.10 ± 0.34 for the reactions (Sn4+ + nH2O ⇌ \({\text{Sn}(\text{OH})}_{n}^{4-n}\) + nH+) where n is 1, 4, 5, and 6, respectively.





Similar content being viewed by others
Notes
No judgments based on values for the formation of Sn(OH)4(aq) can be made because all of these values are unreliable due to experimental concentrations that are at the detection limits.
Ion interaction parameter reported in Rai et al. [2], the reasonableness of this value discussed later.
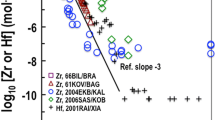
Many other tetravalent ions [Th, U(IV), Np(IV), Pu(IV) and Zr] show much weaker complexes with chloride than those reported by Gajda et al. [10] for Sn(IV). In addition, no multiligand complexes higher than M(Cl)22+ are reported for these other tetravalent ions [5, 6, 8], whereas Gajda et al. [10] report values for complexes up to \({\text{MCl}}_{6}^{2-}\). For examples, 1) the log10 \({\beta }_{1}^{0}\) value reported by Gajda et al. [10] (Table 3) for the formation of SnCl3+ is 3.19, and it varies from 1.5 to 1.8 for tetravalent actinides and Zr and 2) log10 \({\beta }_{2}^{0}\) value of 2.17 [5]), available only for Zr and none of the other tetravalent ions, is over several orders of magnitude lower than the 5.95 value for \({\text{SnCl}}_{2}^{2+}\) reported by Gajda et al. [10]. Rai et al.’s [2] data do not support the formation of strong Sn(IV)-Cl complexes as proposed by Gajda et al. [10] (see Fig. 2).
Although the ε(Zr4+, Cl−) = 0.33 is slightly higher than the recommended value of 0.25 for Th, Hf, and Sn, the Δε of − 0.06 for the generalized reaction [Sn4+ + H2O \(\leftrightharpoons\) SnOH3+] is the same for Th, Zr, Hf, and Sn because ε(ZrOH3+, Cl−) = 0.29 and ε(MOH3+, Cl−) = 0.19 for Th, Hf, and Sn. Therefore the final impact due to chloride interactions is identical in all of these cases.
For details of use with Sn system see [2].
The model presented in this study also provides predicted concentrations that are in close agreement with the SnO2(am) solubility (Fig. not shown), the agreement identical to that presented in Rai et al. [2].
The SIT analyses by Gamsjäger et al. [1] in their Fig. A-4 provide a Δε (mv) = – 9.4 ± 0.7 based on molarity units. The value quoted above is our estimate for molality units.
Estimated by us from Gamsjäger et al.’s [1] Fig. A-11.
Also supported by the experimental data of Vasil’ev and Glavina [19] who observed that Sn-Cl complexes completely dissociate in 0.62 to 2.2 molkg−1 HClO4 solutions. This is contrary to Gajda et al. [10] who propose strong multiple \({\text{SnCl}}_{y}^{4-y}\) complexes (y varying from 1 to 6) in relatively concentrated acids.
Calculated from the log10 K0 of the reaction [Sn4+ + H2(g) \(\leftrightharpoons\) Sn2+ + 2H+] and ΔfG0m of Sn2+. The increase in concentration of Sn4+ results in the decrease in log10 K0 value and a decrease in the calculated ΔfG0m of Sn4+.
The decrease in the Δf \({G}_{\text{m}}^{0}\) of Sn4+ in the reaction [SnO2(cass) + 2H2O \(\leftrightharpoons\) Sn4+ + 4OH−] results in an increase in the value of K0 because [RT ln K0 = Δf \({G}_{\text{m}}^{0}\)(SnO2(cass) + 2 Δf \({G}_{\text{m}}^{0}\)(H2O) − 4 Δf \({G}_{\text{m}}^{0}\)(OH−) − Δf \({G}_{\text{m}}^{0}\)(Sn4+)].
log10 K0 = [(log10 Kc − ΔZ2D + Δε Im) ± [σ(Δε)] Im] for the reactions (Sn4+ + yCl− \(\leftrightharpoons\) \({\text{SnCl}}_{y}^{4-y}\)), where Kc is the concentration constant, Z is the sum of valence squared of ions weighted by the stoichiometric coefficients of the products minus the reactants, Im is the ionic strength in molality units, D = [(0.509 (Im)1/2)/(1 + 1.5 (Im)1/2)], and Δε is the difference between the sum of the ion interaction parameters weighted by the stoichiometric coefficients of the products minus the reactants.
Rai et al. [12] successfully applied the SIT model to a range of ionic strengths extending to as high as 23 molal for [ZrO2(am) + 2H2O + 2OH− \(\rightleftharpoons\) \({\text{Zr}(\text{OH})}_{6}^{2-}]\), where ΔZ2 = 2 and Δε = 0.068.
It was assumed that ε(H+, SnCl5−) = ε(H+, Cl−).
No ion interaction parameters for H+ with doubly charged anions (X2−) are available for comparisons; ε(H+, SnCl62−) value is a rough estimate based on the ε(Na+or K+, X2−) values reported in Rand et al. [8].
References
Gamsjäger, H., Gajda, T., Sangster, J., Saxena, S.K., Voigt, W.: Chemical Thermodynamics of Tin. Chemical Thermodynamics, vol. 12. OECD, Nuclear Energy Agency Data Bank (2012)
Rai, D., Yui, M., Schaef, H.T., Kitamura, A.: Thermodynamic model for SnO2(cr) and SnO2(am) solubility in the aqueous Na+–H+–OH−–Cl−–H2O system. J. Solution Chem. 40, 1155–1172 (2011)
Amaya, T., Chiba, T., Suzuki, K., Oda, C., Yoshikawa, H., Yui, M.: Solubility of Sn(IV) oxide in dilute NaClO4 solution at ambient temperature. Mat. Res. Soc. Symp. Proc. 465, 751–758 (1997)
Oda, C. Amaya, T.: Effects of Ligands on the Solubility of Tin. JNC, Report TN8400 98–001. Japan Nuclear Cycle Development Institute (1998)
Brown, P.L., Curti, E., Grambo, B.: Chemical Thermodynamics of Zirconium. Chemical Thermodynamics, vol. 8. Elsevier, New York (2005)
Guillaumont, R., Fanghanel, T., Fuger, J., Grenthe, I., Neck, V., Palmer, D.A., Rand, M.H.: Update on the Chemical Thermodynamics of Uranium, Neptunium, Plutonium, Americium, and Technetium, vol. 5. Elsevier, New York (2003)
Rai, D., Xia, Y., Hess, N.J., Strachan, D.M., McGrail, B.P.: Hydroxo and chloro complexes/ion-interactions of Hf4+ and the solubility product of HfO2(am). J. Solution Chem. 30, 949–967 (2001)
Rand, M.H., Fuger, J., Grenthe, I., Neck, V., Rai, D.: Chemical Thermodynamics of Thorium. Chemical Thermodynamics, vol. 11. OECD Nuclear Energy Agency (2008)
Shannon, R.D.: Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A32, 751–767 (1976)
Gajda, T., Sipos, P., Gamsjäger, H.: The standard electrode potential of the Sn4+/Sn2+ couple revisited. Monatsh Chem. 140, 1293–1303 (2009)
Huey, C.S., Tartar, H.V.: The stannous-stannic oxidation-reduction potential. J. Am. Chem. Soc. 56, 2585–2588 (1934)
Rai, D., Kitamura, A., Altmaier, M., Rosso, K.M., Sasaki, T., Kobayashi, T.: A thermodynamic model for ZrO2(am) solubility at 25°C in the Ca2+–Na+–H+– Cl−– OH−–H2O system: a critical review. J. Solution Chem. 47, 855–891 (2018)
Rai, D., Kitamura, A., Rosso, K.M., Sasaki, T., Kobayashi, T.: Issues concerning the determination of solubility products of sparingly soluble crystalline solids: solubility of HfO2(cr). Radiochim. Acta 104, 583–592 (2016)
Forbes, G.S., Bartlett, E.P.: The measurement of oxidation potentials at mercury electrodes. I. The Stannic stannous potential. J. Am. Chem. Soc. 56, 2030–2040 (1914)
Wagman, D.D., Evans, W.H., Parker, V.B., Schumm, R.H., Halow, I., Bailey, S.M., Churney, K.L., Nuttall, R.L.: The NBS tables of chemical thermodynamic properties: selected values for inorganic and C1 and C2 organic substances in SI units. J. Phys. Chem. Ref. Data 11, 1–392 (1982)
Despic, A.R., Jovanovic, D.R., Rakic, T.B., Baljkovic, N.A.: Potentiometric study of the tin(II)-tin(IV) redox equilibrium in chloride solution. Bull. Soc. Chim. Beograd 37, 349–362 (1972)
Vasil'ev, V.P., Glavina, S.R., Shorokhova, V.I.: Potentiometric determination of normal Gibbs energy of formation of tin(IV) ion in an aqueous solution. Izv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol 22, 1082–1085 (1979)
Pitzer, K.S., Silvester, L.F.: Thermodynamics of electrolytes. XI. Properties of 3:2, 4:2 and other high-valence types. J. Phys. Chem. 82 (1978)
Vasil’ev, V.P., Glavina, S.R.: State of tin tetrachloride and diammonium hexachlorostannate in perchloric acid solutions. Russ. J. Gen. Chem. 47, 1325–1327 (1977)
Cheng, H.S.: Mössbauer spectroscopic studies of complex equilibrium. Radiochem. Radioanal. Lett. 49, 161–165 (1981)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Retired Laboratory Fellow, Pacific Northwest National Laboratory, Richland, WA (USA).
Appendix A
Appendix A
1.1 A.1 Importance of Sn(IV)/Sn(II) Redox Potentials
Redox potential values for the Sn(IV)/Sn(II) couple in HCl can help determine 1) the critical but previously unknown ε(Sn4, Cl−) value from the Δε (Δε = ε(Sn2+, Cl−) + 2ε(H+, Cl−) − ε(Sn4+, Cl−)) of the reaction (Eq. 9), 2) whether the strong Sn(IV)-Cl complexes, a major cause of disagreement in values for Sn-OH complexes reported by Rai et al. [2] and Gamsjäger et al. [1], recommended by Gamsjäger et al. [1] are consistent with these data, and 3) the solubility product of SnO2(cass) from the Δf\({G}_{\text{m}}^{0}\) value of the species either reported in Gamsjäger et al. [1] or calculated from the Sn(IV)/Sn(II) redox couple potentials, for comparison to the value determined in this study from the solubility data in HCl solutions. Studies involving Sn(IV)/Sn(II) couple potentials must meet the following criteria for the results to be accurate and useful for calculating a ε(Sn4, Cl−) value. The studies need to be conducted
-
only in HCl, a simple electrolyte, rather than mixtures of electrolytes for ease in interpreting the data,
-
at multiple electrolyte concentrations including relatively low ionic strengths (e.g., < ~ 3 mol·kg−1), so that the results can be accurately extrapolated to zero ionic strength using the SIT model,
-
as a function of the electrolyte concentration so that a reliable Δε value for the reaction can be determined,
-
at high enough acid concentration (> ~ 0.5 mol·kg−1) to avoid complications, due to Sn(IV)-OH and Sn(II)-OH complexes, in interpreting the data,
-
at low enough total Sn concentration in experiments to avoid precipitation of SnO2(am), especially at low acidities, and high enough concentrations (> ~ 0.001 mol·kg−1) so that the potentials are well buffered,
-
as a function of Sn concentration and Sn(IV)/Sn(II) ratios close to 1.0 and ideally not varying much beyond 0.1 and 10 so that the potentials are well buffered.
Five studies deal with Sn(IV)/Sn(II) couple potentials (Table 5), but those conducted in only the chloride media are of particular interest for the present. Of these studies, those conducted by Hue and Tartar [11] and Forbes and Bartlett [14] meet most of the criteria laid out above for an ideal study. Although Despic et al.’s [16] study was also conducted in HCl, it was conducted in only 4.0 mol⋅dm−3 HCl and in total ionic strength (due to the presence of Sn(II) and Sn(IV) chlorides) varying to as high as 17.1 mol·kg−1. Therefore, it is not possible to use these data to determine the ε(Sn4, Cl−) values. The other studies are conducted in mixtures of high ionic strength electrolytes (Cl + ClO4) [10, 17] and in (Cl + SO4) [16]) (Table 5).
Gamsjäger et al. [1] extrapolated the redox potentials reported by [11, 14] for the Sn(IV)/Sn(II) couple to zero ionic strength using the SIT model, with which we agree. Their analyses of Forbes and Bartlett’s [14] experimental data in chloride concentrations ranging from 1.02 to 5.59 mol·kg−1 provides Δε (mv) = − 8.23 ± 0.61Footnote 9 (or Δε (mol·kg−1) = (− Δε (mv))/29.58 = [− (− 8.23 ± 0.61)]/ 29.58 = 0.28 ± 0.02) for the reaction in Eq. 9.
The Δε for Eq. 9 is represented in Eq. 10.
By rearranging Eq. 10, we get Eq. 11.
By substituting into Eq. 11 the known values of Δε = 0.28 ± 0.02 mol·kg−1 [calculated by Gamsjäger et al. [1] using SIT analyses of Forbes and Bartlett’s [14] data], and ε(Sn2+, Cl−) = 0.14 ± 0.1 mol·kg−1 and ε(H+, Cl−) = 0.12 ± 0.01 mol·kg−1 from [1]), we calculate the value of ε(Sn4+, Cl−) = 0.10 ± 0.1 mol·kg−1.
Gamsjäger et al.’s [1] SIT analyses of Huey and Tartar’s [11] data provide Δε = − 0.14 ± 0.05 mol·kg−1 (for Eq. 9),Footnote 10 where [Δε = ε(Sn2+, Cl−) – ε(Sn4+, Cl−)] because the value of 2ε(H+, Cl−) is already included in the analyses. These data provide a value of ε(Sn4+, Cl−) = 0.28 ± 0.11 mol·kg−1.
An average value of ε(Sn4+, Cl−) = 0.19 ± 0.11 mol·kg−1 is calculated from the values based on the analyses of Forbes and Bartlett’s [14] and Huey and Tartar’s [11] data discussed above; these are the only data sets where such analyses are possible. Considering the uncertainties involved in the average value, it is close to the value (0.25 mol·kg−1) based on the relationship of ε(M4+, Cl−) values to 1/r2 (Fig. 3) and the value that explains Rai et al.’s [2] SnO2(cass) solubility data in HCl concentrations extending to 1.0 mol·kg−1. These extensive data show that chloride interactions with Sn4+ are rather weak and not consistent with the extremely strong Sn(IV)-Cl complexes recommended by Gamsjäger et al. [1].
1.2 A.2 Sn(IV)-Cl Complexes Reported in Gamsjäger et al. [1]
Gamsjäger et al. [1] chose to use Gajda et al.’s [10] data for \({\text{SnCl}}_{y}^{4-y}\) complexes (Table 3). The equilibrium constants for the formation of \({\text{SnCl}}_{y}^{4-y}\) they report are extremely high and are based on extrapolations of the data from ionic strengths as high as 12.18 molal to zero ionic strength using the SIT model. There are many uncertainties associated with the equilibrium constant values thus calculated. (1) The SIT model is generally considered to be accurate when applied to ionic strengths of < ~ 3.0 molal, and all of these data were obtained at ionic strengths much higher than that (5.62 to 12.18). (2) Sn4+ is a high valence ion, the thermodynamics of which are complicated by both the very strong specific interactions and the possible ion pairing in dilute solution and by an effective possible redissolution of these complexes into the bare ions (resulting from a rapid decrease in values for the activity coefficients of the highly charged bare ion [18]) that can occur at high concentrations. This possible redissolution of \({\text{SnCl}}_{y}^{4-y}\) complexesFootnote 11 at higher electrolyte concentrations means that the bare ion (Sn4+) can become increasingly important at higher ionic strengths, with the net result of lowering the Δf\({G}_{\text{m}}^{0}\)(Sn4+) valueFootnote 12 and increasing the solubility product of SnO2(cass).Footnote 13 3) Because (log10 K0 = log10 Kc – ΔZ2D + ΔεIm),Footnote 14 under special circumstances where ΔZ2 and Δε are zero or nearly zero (neither highly negative or positive) it is possible to use the SIT model to very high ionic strengths[12].Footnote 15 However, in the case of Sn(IV)-Cl complexes, the ΔZ2 varies from − 8 to − 20 and Δε varies from − 0.26 to − 0.67 (Table 3), clearly relatively highly negative and not near zero and would, for example, result in − 8.16 (ΔεIm = − 0.67 × 12.18) orders of magnitude extrapolation corrections to the equilibrium constant for the formation of \({\text{Sn}(\text{Cl})}_{6}^{2-}\) due to ion interaction parameters alone. 4) The Δε values they fitted represent the data well but this does not mean that these values are meaningful or that they can be used to accurately extrapolate the equilibrium constants to zero ionic strength. In fact the Δε values they report (Table 3) in combination with ε(Sn4+, ClO4−) = 0.7 ± 0.2 and ε(H+, Cl−) = 0.12 ± 0.01 reported in [1] provide values for [ε(H+, \({\text{SnCl}}_{y}^{4-y}\)) for y 4 to 6] that are very high and extremely inconsistent with the SIT model. For examples, the calculated value for ε(\({\text{SnCl}}_{4}^{0}\), H+ or \({\text{SnCl}}_{4}^{0}\), ClO4) is 0.54 whereas for such neutral species the NEA (e.g., Rand et al. [8]) recommends a value of 0.00; ε(H+, \({\text{SnCl}}_{5}^{-}\)) is 0.7 whereas it is expected to be near 0.12Footnote 16; and ε(H+, \({\text{SnCl}}_{6}^{2-}\)) is 0.75 whereas it is expected to be < 0.0.Footnote 17 A cursory glance might suggest that the differences noted above are not very large. However, in all the cases noted above, the differences are > 0.5, and when this number is multiplied by high ionic strength values (e. g., 12 mol·kg–1), it would result in corrections of over 6 orders of magnitude to the equilibrium constant.
In addition to these uncertainties, the reported values of equilibrium constants for the formation of \({\text{SnCl}}_{y}^{4-y}\) are clearly extremely high and inconsistent with:
-
the extensive data of Forbes and Bartlett [14], Huey and Tartar [11], and Rai et al. [2] which show that Sn(IV)-Cl interactions are rather weak and can be explained by relatively low ion interaction parameters (ε(Sn4+, Cl−) = 0.25)
-
the expected observed behavior for many other tetravalent ions in the presence of chloride, which do not show such strong complexes with chloride, and their behavior can be predicted based on ion interaction parameters (Fig. 3).
-
the observed solubility of SnO2(cass) in the presence of HCl where the use of complexation constant values for \({\text{SnCl}}_{y}^{4-y}\) from [1] would result in predicted Sn concentrations that are over four orders of magnitude higher than the observed (Fig. 2).
-
the observed Sn(IV)-Cl complexation constant values (Cheng [20]Footnote 18) obtained in relatively low ionic strength solutions that are relatively orders of magnitude lower than those recommended in Gamsjäger et al. [1]
In conclusion, the Sn(IV)-Cl complexation constants recommended in Gamsjäger et al. [1] cannot be correct. Therefore, other values [Δf\({G}_{\text{m}}^{0}\)(Sn4+), E0(Sn4+/ Sn2+), and \({K}_{\text{so}}^{0}\)(SnO2(cass)] based on these constants are also unreliable.
1.3 A.3 Sn(IV)/Sn(II) Redox Potentials and the Solubility Product of SnO2(cass)
Of all available data for the Sn4+/Sn2+ couple (Table 5), the only data that are consistent with the SIT model and obtained in single electrolyte solutions (e.g., HCl) extending to low ionic strengths are those of Huey and Tartar [11] and Forbes and Bartlett [14]. Gamsjäger et al.’s [1] analyses of these data using the SIT model provide E0/mV values of (145.1 ± 1.8) and (207.7 ± 1.4) for the reaction (Eq. 9), respectively, with an average of these values of 176.4 ± 44.3. This data in combination with Δf \({G}_{\text{m}}^{0}\) values of Sn2+, SnO2(cass), H2O, and OH− reported in Gamsjäger et al. [1] result in a log10 \({K}_{\text{so}}^{0}\) (Eq. 1) of (− 64.59 ± 1.50), which is similar to the value (− 63.57 ± 0.30) determined in this study based on extensive solubility data but is drastically different than (− 71.60 ± 0.68) recommended by Gamsjäger et al. [1].
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rai, D. Thermodynamic Data for Sn(IV) Dioxides and Hydroxido Complexes: A Critical Review. J Solution Chem 51, 1169–1186 (2022). https://doi.org/10.1007/s10953-022-01188-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-022-01188-6