Abstract
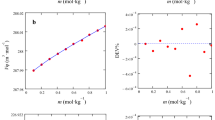
In accordance with the ideal associated solution model, all departures from ideality of a mixture of two interacting non-electrolytes A and B can be accounted for in terms of the ideal properties of the ternary system comprising A, B, and their 1:1 complex AB co-existing at equilibrium. This article shows that the ideal associated solution model in conjunction with an ideal viscosity equation put forward by Schutte et al. (Can J Chem 67:446–448, 1989) can describe the viscosities of highly non-ideal binary mixtures of chloroform (A) and diisopropyl ether (B) over the entire composition range very accurately. In this context, the applicability of Grunberg–Nissan equation (Grunberg and Nissan in: Nature 164:799–800, 1949) has also been tested to describe the composition dependence of the viscosities of chloroform (A) + diisopropyl ether (B) system. The quality of fit in the former case, however, is somewhat better compared to that of the later. Further, the former model provides an equilibrium speciation of A, B, and AB in the chloroform (A) + diisopropyl ether (B) system thus offering a complete description of the equilibrium composition in the mixtures. This study recommends the analysis of the viscosity of mixtures of non-electrolytes where there is convincing evidence for the existence of a complex of the type mentioned above on the basis of the ideal associated solution model.


Similar content being viewed by others
References
Grunberg, L., Nissan, A.H.: Mixture law for viscosity. Nature 164, 799–800 (1949)
Grunberg, L.: The viscosity of regular solutions systems involving carbon tetrachloride, benzene and cyclohexane. Trans. Faraday Soc. 50, 1293–1303 (1954)
Schutte, R.P., Liu, T.C., Hepler, L.G.: Viscosities of mixtures of chloroform + trimethylamine: analysis in terms of three components (A, B, and AB). Can. J. Chem. 67, 446–448 (1989)
Fenby, D.V., Hepler, L.G.: Calorimetric investigations of hydrogen bond and charge transfer complex. Chem. Soc. Rev. 3, 193–207 (1974)
Kutsyk, A.M., Ilchenko, O.O., Nikonova, V.V., Obukhovsky, V.V.: Mixing dynamics of diethyl ether and chloroform. J. Mol. Liq. 339, 116687 (2021)
Kutsyk, A., Ilchenko, O., Pilgun, Y., Obukhovsky, V., Nikonova, V.: Complex formation in liquid diethyl ether–chloroform mixtures examined by 2D correlation MID-IR spectroscopy. J. Mol. Str. 1124, 117–124 (2016)
Ilchenko, O.O., Nikonova, V.V., Kutsyk, A.M., Obukhovsky, V.V.: Quantitative analysis of complex formation in acetone–chloroform and ethyl acetate–cyclohexane solutions. Ukr. J. Phys. 59, 268–275 (2014)
Ilchenko, O.O., Kutsyk, A.M., Obukhovsky, V.V.: Study of complexation in acetone–chloroform mixtures by infrared spectroscopy. J. At. Mol. Physics 2014, 106178 (2014)
Marigliano, A.C.G., del Campos, V.V., Fernandez, L., Rolda, M.L., Solimo, H.N.: Spectroscopic and thermodynamic evidence of dimer and trimer hydrogen bonded complex formation between chloroform and 2-butanone. Excess molar enthalpy for the chloroform + 2-butanone binary system at 303 K. J. Phys. Chem. B 117, 5121–5128 (2013)
Smith, J.F.: Calculations of speciation of associated solutions from vapour pressure–composition data fluid. Phase Equilib. 78, 219–227 (1992)
Barta, L., Kooner, Z.S., Hepler, L.G., Roux-Desganges, G., Grolier, J.-P.E.: Thermal and volumetric properties of chloroform + dimethylsulfoxide: thermodynamic analysis using ideal associated solution model. J. Solution Chem. 18, 663–673 (1989)
Hepler, L.G., Kooner, Z.S., Roux-Desgranges, G., Grolier, J.-P.E.: Thermal and volumetric properties of chloroform + trietylamine mixtures and the ideal associated solution model of complex formation. J. Solution Chem. 14, 579–594 (1985)
Dohnal, V., Costas, M.: Thermodynamics of complex formation in chloroform–oxygenated solvent mixtures. J. Solution Chem. 25, 635–656 (1996)
Naessems, R.M., Clara, R.A., Marigliano, A.C.G.: Thermophysical properties for the [chloroform + di-isopropyl ether (DIPE) + ethanol] ternary system at (298.15 ± 0.01) K. Chem. Data Coll. 28, 100428 (2020)
Ouerfelli, N., Barhoumi, Z., Iulian, O.: Viscosity Arrhenius activation energy and derived partial molar properties in 1,4-dioxane +water binary mixtures from 293.15 to 323.15 K. J. Solution Chem. 41, 458 (2012)
Reddy, G.S., Subbaiah, M.V., Boddu, V.M., Krishnaiah, A.: Thermo physical properties of binary mixtures of n-butylamine with alkyla acetates at 303.15 K. J. Mol. Liq. 141, 94 (2008)
Chen, H.-W., Tu, C.-H.: Densities, viscosities, and refractive indices for binary and ternary mixtures of diisopropyl ether, ethanol, and 2,2,4-trimethylpentane. J. Chem. Eng. Data 51, 261 (2006)
Acknowledgements
The authors acknowledge the financial support by the Presidency University, Kolkata, India.
Author information
Authors and Affiliations
Contributions
Conceptualization: [BD]; Methodology: [SB]; Formal analysis and investigation: [SB]; Writing—original draft preparation: [SB]; Writing—review and editing: [BD]; Funding acquisition: [BD]; Resources: [BD]; Supervision: [BD].
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bajpayee, S., Das, B. Speciation in Chloroform + Diisopropyl Ether Binaries in the Light of the Ideal Associated Solution Model Using Viscometric Data. J Solution Chem 51, 126–134 (2022). https://doi.org/10.1007/s10953-022-01142-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-022-01142-6