Abstract
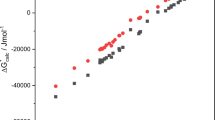
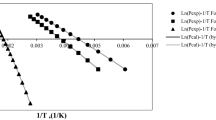
The paper studies quadratic solvation models (QSMs) applied to the numerical simulation of the vapor pressures of solvent systems with a salt effect. Two useful general quadratic solvation relationships are presented within an integration framework, incorporating cumulative physical indices for components and boundary constraints, in conjunction with the vapor pressures of monomolecular fluids calculated from Antoine, Senol, Frost-Kalkwarf and Xiang-Tan equations. Literature data for the vapor pressures of 18 diverse binary (solvent + salt) and ternary (solvent 1 + solvent 2 + salt 3) vapor–liquid equilibrium systems are subjected to the statistical analysis of QSMs via a logarithmic-ratio objective function and cumulative frequency distribution. Essentially, the examined QSMs with twelve (QSM12) and six (QSM6) adjustable coefficients are quite accurate in yielding overall design factors (Fod) lower than 1.015 and 1.08, respectively. The concentration-dependent model (CM) also simulates precisely the observed data with Fod = 1.013 as far as salt effects are concerned. QSM12 models have proven reasonably successful in predicting the vapor pressures of ternary systems with a mean deviation of 2.2%.






Similar content being viewed by others
Abbreviations
- \(C_{{\text{s}}}\) :
-
Salt concentration (wt %)
- \(cu\) :
-
Cumulative descriptor
- \(\overline{e}\) :
-
Mean relative error, \( \bar{e}{\text{ = }}\left( {{{{\text{100}}} \mathord{\left/ {\vphantom {{{\text{100}}} {\text{N}}}} \right. \kern-\nulldelimiterspace} {\text{N}}}} \right)\sum\nolimits_{{i = 1}}^{N} {\left| {{{\left( {P_{{i{\text{,obs}}}} - P_{{i{\text{,mod}}}} } \right)} \mathord{\left/ {\vphantom {{\left( {P_{{i{\text{,obs}}}} - P_{{i{\text{,mod}}}} } \right)} {P_{{i{\text{,obs}}}} }}} \right. \kern-\nulldelimiterspace} {P_{{i{\text{,obs}}}} }}} \right|} \) (\(\%\))
- \(F\) :
-
Concentration dependent factor
- \(F_{{\text{m}}}\) :
-
Model normalization factor
- \(F_{{{\text{od}}}}\) :
-
Overall design factor
- \(F_{{\text{s}}}\) :
-
Safety factor
- \(N\) :
-
Number of observations
- \(n_{i}\) :
-
Stoichiometric number of the ion in the salt structure
- \(P_{i}\) :
-
Partial pressure of a component in the solvent mixture (kPa)
- \(P\) :
-
Vapor pressure of (solvent + salt) system (kPa)
- \(P_{0}\) :
-
Vapor pressure of a pure solvent (kPa)
- \(P_{{\text{c}}}\) :
-
Critical vapor pressure of a pure solvent (kPa)
- \(P_{{\text{t}}}^{{\prime}}\) :
-
Total vapor pressure of a solvent mixture involving a salt (kPa)
- \(r\) :
-
Crystal ionic radius of the ion (nm)
- \(r^{{\prime}}\) :
-
The normalized reciprocal of the crystal ionic radius (nm−1)
- \(R_{{\text{D}}}\); \(R_{{\text{D}}}^{{\prime}}\) :
-
Molar refractivity of the ion and its normalized value (cm3·mol−1)
- \(S\) :
-
Standard deviation
- \(T\) :
-
Temperature (\({\text{K}}\))
- \(T_{{\text{c}}}\) :
-
Critical temperature of a pure solvent (K)
- \(t\) :
-
Student’s t parameter
- \(V\) :
-
Molar volume of the component (dm3·mol−1)
- \(v\); \(v^{{\prime}}\) :
-
Volume occupied by the ion and its normalized value (nm3)
- w solv :
-
Mass fraction of solvent
- \(X\) :
-
Objective function of model reliability
- \(\overline{X}\) :
-
Mean of objective function
- \(x_{i}\) :
-
Mole fraction of the component in the liquid phase
- \(x_{W}^{{\prime}}\) :
-
The argument of the Lambert W function
- \(Y\) :
-
Independent variable of objective function
- \(y_{i}\) :
-
Mole fraction of the component in the vapor phase
- \(z\) :
-
The charge of the ion
- α; α*:
-
Solvatochromic parameters
- β; β*:
-
Solvatochromic parameters
- δ; δ*:
-
Solvatochromic parameters
- δH; δH*:
-
Hildebrand solubility parameter (MPa0.5)
- ϕ; ϕ0 :
-
Fugacity coefficients of the component
- γ :
-
Activity coefficient of the component
- π; π*:
-
Solvatochromic parameters
- σ :
-
Root mean square deviation, \( \sigma {\text{ = }}\left[ {{{\sum\nolimits_{{i = 1}}^{N} {\left( {Y_{{i,{\text{obs}}}} - Y_{{i,{\text{mod}}}} } \right)^{2} } } \mathord{\left/ {\vphantom {{\sum\nolimits_{{i = 1}}^{N} {\left( {Y_{{i,{\text{obs}}}} - Y_{{i,{\text{mod}}}} } \right)^{2} } } N}} \right. \kern-\nulldelimiterspace} N}} \right]^{{0.5}} \)
- σ p :
-
Softness parameter of the ion
- desn:
-
Design property
- mod:
-
Modeled property
- obs:
-
Observed property
- s:
-
Salt
References
Pereiro, A.B., Araújo, J.M.M., Esperança, J.M.S.S., Marrucho, I.M., Rebelo, L.P.N.: Ionic liquids in separations of azeotropic systems: a review. J. Chem. Thermodyn. 46, 2–28 (2012)
Hwang, I.-C., Kwon, R.-H., Park, S.-J.: Azeotrope breaking for the system ethyl tert–butyl ether (ETBE) + ethanol at 313.15 K and excess properties at 298.15 K for mixtures of ETBE and ethanol with phosphonium–based ionic liquids. Fluid Phase Equilib. 344, 32–37 (2013)
Li, X.-M., Shen, C., Li, C.-X.: Effect of alkanolammonium formates ionic liquids on vapour−liquid equilibria of binary systems containing water, methanol, and ethanol. J. Chem. Thermodyn. 53, 167–175 (2012)
Furter, W.F.: Thermodynamic Behavior of Electrolytes in Mixed Solvents. Advances in Chemistry Series. American Chemical Society, Washington, DC (1976)
Mock, B., Evans, L.B., Chen, C.C.: Thermodynamic representation of phase equilibria of mixed-solvent electrolyte systems. AIChE J. 22, 1655–1664 (1986)
Uhrig, G., Ji, X., Maurer, G.: Vapor–liquid equilibrium in systems (water + organic solvent + salt) at low water concentrations but high ratios of salt to water: experimental results and modeling. Fluid Phase Equilib. 228–229, 5–14 (2005)
Apelblat, A., Korin, E.: The molar enthalpies of solution and vapour pressures of saturated aqueous solutions of some cesium salts. J. Chem. Thermodyn. 38, 152–157 (2006)
Kumar, A.: Effect on vapor–pressure equilibria: a review of correlations and predictive models. Sep. Sci. Technol. 28, 1799–1818 (1993)
Patel, B.H., Paricaud, P., Galindo, A., Maitland, G.C.: Prediction of the salting-out effect of strong electrolytes on water + alkane solutions. Ind. Eng. Chem. Res. 42, 3809–3823 (2003)
Wang, J.-F., Li, C.-X., Wang, Z.-H., Li, Z.-J., Jiang, Y.-B.: Vapor pressure measurement for water, methanol, ethanol, and their binary mixtures in the presence of an ionic liquid 1–ethyl–3–methylimidazolium dimethylphosphate. Fluid Phase Equilib. 255, 186–192 (2007)
Sandler, S.I.: Models for Thermodynamic and Phase Equilibria Calculations (Chemical Industries). Marcel Dekker, New York (1993)
Sandler, S.I.: Chemical, Biochemical, and Engineering Thermodynamics. Wiley, New York (2006)
Prausnitz, J.M., Lictenthaler, R.N., de Azevedo, E.G.: Molecular Thermodynamics of Fluid-Phase Equilibria, 3rd edn. Prentice-Hall Inc., New Jersey (1999)
Prausnitz, J.M., Anderson, T., Grens, E., Eckert, C., Hsieh, R., O'Connell, J.P.: Computer Calculations for Multicomponent Vapor–Liquid and Liquid–Liquid Equilibria. Prentice-Hall Inc., Englewood Cliffs (1980)
Kikic, I., Fermeglia, M., Rasmussen, P.: UNIFAC prediction of vapor–liquid equilibria in mixed solvent–salt systems. Chem. Eng. Sci. 46, 2775–2780 (1991)
Macedo, E.A., Skovborg, P., Rasmussen, P.: Calculation of phase equilibria for solutions of strong electrolytes in solvent–water mixtures. Chem. Eng. Sci. 45, 875–882 (1990)
Mohs, A., Gmehling, J.: A revised LIQUAC and LIFAC Model (LIQUAC*/LIFAC*) for the prediction of properties of electrolyte containing solutions. Fluid Phase Equilib. 337, 311–322 (2013)
Gani, R., Muro-Suñé, N., Sales-Cruz, M., Leibovici, C., O’Connell, J.P.: Mathematical and numerical analysis of classes of property models. Fluid Phase Equilib. 250, 1–32 (2006)
Mahdoui, N.B., Artal, M., Hichri, M., Lafuente, C.: Volumetric behavior and vapor–liquid equilibrium of dimethyl disulfide + n-alkanol binary mixtures. J. Solution Chem. 48, 1–14 (2019)
Zhou, T., McBride, K., Zhang, X., Qi, Z., Sundmacher, K.: Integrated solvent and process design exemplified for a Diels–Alder reaction. AIChE J. 61, 147–158 (2015)
Simoni, L.D., Brennecke, J.F., Stadtherr, M.A.: Asymmetric framework for predicting liquid-liquid equilibrium of ionic liquid-mixed-solvent systems. 1. Theory, phase stability analysis, and parameter estimation. Ind. Eng. Chem. Res. 48, 7246–7256 (2009)
Simoni, L.D., Chapeaux, A., Brennecke, J.F., Stadtherr, M.A.: Asymmetric framework for predicting liquid-liquid equilibrium of ionic liquid-mixed-solvent systems. 2. Prediction of ternary systems. Ind. Eng. Chem. Res. 48, 7257–7265 (2009)
Poling, B.E., Prausnitz, J.M., O’Conell, J.P.: The Properties of Gases and Liquids, 5th edn. McGraw-Hill, New York (2001)
Reid, R.C., Prausnitz, J.M., Poling, B.E.: The Properties of Gases and Liquids, 4th edn. McGraw-Hill, New York (1987)
Bejarano, A., Poveda, L.J., de la Fuente, J.C.: Supplementary vapor pressure data of the glycol ethers, 1-methoxy-2-propanol, and 2-methoxyethanol at a pressure range of (15 to 177) kPa. J. Chem. Thermodyn. 53, 114–118 (2012)
Růžička, K., Majer, V.: Simple and controlled extrapolation of vapor pressures toward the triple point. AIChE J. 42, 1723–1740 (1996)
Luis, A., Forero, G., Jorge, A., Velásquez, J.: Wagner liquid–vapour pressure equation constants from a simple methodology. J. Chem. Thermodyn. 43, 1235–1251 (2011)
Huber, M.L., Laesecke, A., Friend, D.G.: Correlation for the vapor pressure of mercury. Ind. Eng. Chem. Res. 45, 7351–7361 (2006)
Godavarthy, S.S., Robinson Jr., R.L., Gasem, K.A.M.: SVRC–QSPR model for predicting saturated vapor pressures of pure fluids. Fluid Phase Equilib. 246, 39–51 (2006)
Nieto-Draghi, C., Fayet, G., Creton, B., Rozanska, X., Rotureau, P., de Hemptinne, J.C., Ungerer, P., Rousseau, B., Adamo, C.: A general guidebook for the theoretical prediction of physicochemical properties of chemicals for regulatory purposes. Chem. Rev. 115, 13093–13164 (2015)
Bilde, M., Barsanti, K., Booth, M., Cappa, C.D., Donahue, N.M., Emanuelsson, E.U., McFiggans, G., Krieger, U.K., Marcolli, C., Topping, D., Ziemann, P., Barley, M., Clegg, S., Dennis-Smither, B., Hallquist, M., Hallquist, Å.M., Khlystov, A., Kulmala, M., Mogensen, D., Percival, C.J., Pope, F., Reid, J.P., da Silva, M.A.V.R., Rosenoern, T., Salo, K., Soonsin, V.P., Yli-Juuti, T., Prisle, N.L., Pagels, J., Rarey, J., Zardini, A.A., Riipinen, I.: Saturation vapor pressures and transition enthalpies of low-volatility organic molecules of atmospheric relevance: from dicarboxylic acids to complex mixtures. Chem. Rev. 115, 4115–4156 (2015)
Katritzky, A.R., Kuanar, M., Slavov, S., Hall, C.D., Karelson, M., Kahn, I., Dobchev, D.A.: Quantitative correlation of physical and chemical properties with chemical structure. Utility for prediction. Chem. Rev. 110, 5714–5789 (2010)
Katritzky, A.R., Slavov, S.H., Dobchev, D.A., Karelson, M.: Rapid QSPR model development technique for prediction of vapor pressure of organic compounds. Comput. Chem. Eng. 31, 1123–1130 (2007)
Senol, A.: Solvation–based vapour pressure model for (solvent + salt) systems in conjunction with the Antoine equation. J. Chem. Thermodyn. 67, 28–39 (2013)
Senol, A.: Solvation–based modeling vapor pressures of (solvent + salt) systems with the application of Cox equation. Fluid Phase Equilib. 361, 155–170 (2014)
Senol, A.: LSER-based modeling vapor pressures of (solvent + salt) systems by application of Xiang-Tan equation. Chin. J. Chem. Eng. 23, 1374–1383 (2015)
Senol, A.: Modeling vapor pressures of solvent systems with and without a salt effect. An extension of the LSER approach. J. Chem. Thermodyn. 81, 1–15 (2015)
Perry, R.H., Green, D.W., Maloney, J.O.: Perry’s Chemical Engineers’ Handbook, 7th edn. McGraw-Hill, New York (1997)
Ji, L., Li, P., Schoen, R., Simon, L.: Handbook of Geometric Analysis. International Press, Somerville (2008)1
Kostrikin, A.I., Manin, Y.I.: Linear Algebra and Geometry. Gordon and Breach Science Publishers, Amsterdam (1997)
Bhatti, M.A.: Practical Optimization Methods. Springer, New York (2000)
Giannessi, F., Rapcsák, T., Komlósi, S.: New Trends in Mathematical Programming. Springer, Dordrecht (1998)
Katritzky, A.R., Fara, D.C., Yang, H.F., Tämm, K., Tamm, T., Karelson, M.: Quantitative measures of solvent polarity. Chem. Rev. 104, 175–198 (2004)
Marcus, Y.: The properties of organic liquids that are relevant to their use as solvating solvents. Chem. Soc. Rev. 22, 409–416 (1993)
Reichardt, C.: Solvatochromic dyes as solvent polarity indicators. Chem. Rev. 94, 2319–2358 (1994)
Kamlet, M.J., Doherty, R.M., Abraham, M.H., Marcus, Y., Taft, R.W.: Linear solvation energy relationships. 46. An improved equation for correlation and prediction of octanol/water partition coefficients of organic nonelectrolytes (including strong hydrogen bond donor solutes). J. Phys. Chem. 92, 5244–5255 (1988)
Marcus, Y.: Linear solvation energy relationships: correlation and prediction of the distribution of organic solutes between water and immiscible organic solvents. J. Phys. Chem. 95, 8886–8891 (1991)
Marcus, Y., Kamlet, M.J., Taft, R.W.: Linear solvation energy relationships: standard molar Gibbs free energies and enthalpies of transfer of ions from water into nonaqueous solvents. J. Phys. Chem. 92, 3613–3622 (1988)
Barton, A.F.M.: Solubility parameters. Chem. Rev. 75, 731–753 (1975)
Soffer, N., Bioemendal, M., Marcus, Y.: Molar refractivities of tetra–n–alkylammonium salts and ions. J. Chem. Eng. Data 3, 43–46 (1988)
Marcus, Y.: On enthalpies of hydration, ionization potentials and the softness of ions. Thermochim. Acta 104, 389–394 (1986)
Reid, R.C., Prausnitz, J.M., Sherwood, T.K.: The Properties of Gases and Liquids, 3rd edn. McGraw-Hill, New York (1977)
Xiang, H.W., Tan, L.C.: A new vapor–pressure equation. Int. J. Thermophys. 15, 711–724 (1994)
Xiang, H.W.: Vapor pressures from a corresponding–states principle for a wide range of polar molecular substances. Int. J. Thermophys. 22, 919–932 (2001)
Frost, A.A., Kalkwarf, D.R.: A semi-empirical equation for the vapor pressure of liquids as a function of temperature. J. Chem. Phys. 21, 264–267 (1953)
Leibovici, C.F., Nichita, D.V.: A quasi-analytical solution of Frost–Kalkwarf vapor pressure equation. Comput. Chem. Eng. 58, 378–380 (2013)
Wagner, W.: New vapour pressure measurements for argon and nitrogen and a new method for establishing rational vapour pressure equations. Cryogenics 13, 470–482 (1973)
Nichols, T., Utgikar, V.: Wagner equation predicting entire curve for pure fluids from limited VLE data. Critical point and four Antoine analytic points. Fluid Phase Equilib. 460, 1–16 (2018)
McGarry, J.: Correlation and prediction of the vapor pressures of pure liquids over large pressure ranges. Ind. Eng. Chem. Proc. Des. Dev. 22, 313–322 (1983)
Kolář, P., Nakata, H., Tsuboi, A., Wang, P., Anderko, A.: Measurement and modeling of vapor–liquid equilibria at high salt concentrations. Fluid Phase Equilib. 228–229, 493–497 (2005)
Fu, J.: Salt effect on vapor–liquid equilibria for binary systems of propanol/CaCl2 and butanol/CaCl2. Fluid Phase Equilib. 237, 219–223 (2005)
Fu, J.: Isobaric vapor liquid equilibrium for the methanol + ethanol + water + ammonium bromide system. J. Chem. Eng. Data 43, 403–408 (1998)
Vercher, E., Orchillés, A.V., Miguel, P.J., González-Alfaro, V., Martínez-Andreu, A.: Isobaric vapor–liquid equilibria for acetone + methanol + lithium nitrate at 100 kPa. Fluid Phase Equilib. 250, 131–137 (2006)
Vercher, E., Vázquez, M.I., Martínez-Andreu, A.: Isobaric vapor–liquid equilibria for 1–propanol + water + lithium nitrate at 100 kPa. Fluid Phase Equilib. 202, 121–132 (2002)
Nie, N., Zheng, D., Dong, L., Li, Y.: Thermodynamic properties of the water + 1-(2-hydroxylethyl)-3-methylimidazolium chloride system. J. Chem. Eng. Data 57, 3598–3603 (2012)
Safarov, J.T.: Vapor pressures of lithium bromide or lithium chloride and ethanol solutions. Fluid Phase Equilib. 243, 38–44 (2006)
Yang, C., Ma, S., Yin, X.: Organic salt effect of tetramethylammonium bicarbonate on the vapor–liquid equilibrium of the methanol–water system. J. Chem. Eng. Data 56, 3747–3751 (2011)
Himmelblau, D.M., Riggs, J.: Basic Principles and Calculations in Chemical Engineering, 8th edn. Prentice-Hall Inc., Englewood Cliffs, New Jersey (2012)
Kuo, S.S.: Computer Applications of Numerical Methods. Addison-Wesley, London (1972)
Johnson, N.L., Leone, F.C.: Statistics and Experimental Design in Engineering and the Physical Sciences. Wiley, New York (1964)
Zwillinger, D., Kokoska, S.: CRC Standard Probability and Statistics Tables and Formulae. CRC Press, New York (2010)
Senol, A.: Optimum mass transfer area in a pilot plant packed distillation column. J. Chem. Eng. Jpn. 39, 1265–1275 (2006)
Kontogeorgis, G.M., Folas, G.K.: Thermodynamic Models for Industrial Applications. From Classical and Advanced Mixing Rules to Association Theories. Wiley, London (2010)
Kontogeorgis, G.M., Tsivintzelis, I., Michelsen, M.L., Stenby, E.H.: Towards predictive association theories. Fluid Phase Equilib. 301, 244–256 (2011)
Senol, A.: Modeling liquid–liquid equilibrium of quaternary systems with Integrated Quadratic Solvation Relationship (IQSR) in conjunction with the boundary constraints identified by ternary subsystems. Fluid Phase Equilib. 478, 58–74 (2018)
Olsen, R., Kvamme, B., Kuznetsova, T.: Hydrogen bond lifetimes and statistics of aqueous mono-, di- and tri-ethylene glycol. AIChE J. 63, 1674–1689 (2017)
Hansen, C.M.: Hansen Solubility Parameters. A User’s Handbook, 2nd edn. CRC Press, Boca Raton (2007)
Andreatta, A.E., Charnley, M.P., Brennecke, J.P.: Using ionic liquids to break the ethanol−ethyl acetate azeotrope. ACS Sustain. Chem. Eng. 3, 3435–3444 (2015)
Endo, S., Pfennigsdorff, A., Goss, K.-U.: Salting-out effect in aqueous NaCl solutions: trends with size and polarity of solute molecules. Environ. Sci. Technol. 46, 1496–1503 (2012)
Endo, S., Droge, S.T.J., Goss, K.-U.: Polyparameter linear free energy models for polyacrylate fiber−water partition coefficients to evaluate efficiency of solid-phase microextraction. Anal. Chem. 83, 1394–1400 (2011)
Abraham, M.H., Ibrahim, A., Zissimos, A.M.: Determination of sets of solute descriptors from chromatographic measurements. J. Chromatogr. A 1037(1–2), 29–47 (2004)
Abraham, M.H.: Scales of solute hydrogen-bonding: their construction and application to physicochemical and biochemical processes. Chem. Soc. Rev. 22(1), 73–83 (1993)
Oleszek-Kudlak, S., Grabda, M., Shibata, E., Eckert, F., Nakamura, T.: Application of the conductor-like screening model for real solvents for prediction of the aqueous solubility of chlorobenzenes depending on temperature and salinity. Environ. Toxicol. Chem. 24(6), 1368–1375 (2005)
Acknowledgements
The constructive proposals of the referees are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supporting information
The coefficients of Eqs. 6–9, complete set of the regression coefficients of Eqs. 10–16and the deviation statistics of model reliability analysis relating to systems I–XVI areprovided in the supplementary Tables S1–S11. This material is available free of charge viathe Internet at the Web site of the journal. (DOCX 375 kb)
Rights and permissions
About this article
Cite this article
Senol, A. Estimation of Vapor Pressures of Solvent + Salt Systems with Quadratic Solvation Relationships. J Solution Chem 49, 559–582 (2020). https://doi.org/10.1007/s10953-020-00983-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-020-00983-3