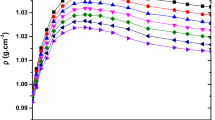
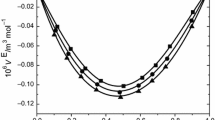
Abstract
Density and speed of sound measurements have been performed, at atmospheric pressure, using an Anton Paar digital vibrating tube densitometer for pure ethanol, 1-octanol, n-hexane, furan and eugenol, from 278.15 to 323.15 K and for the binary mixtures of furan + ethanol, furan + 1-octanol, eugenol + 1-octanol and eugenol + n-hexane from 278.15 to 323.15 K. Excess molar volumes were calculated and compared. The Redlich–Kister correlation was used to correlate the data. In order to identify the most important molecular interaction contributing to the excess molar volume, the Prigogine–Flory–Patterson theory was applied to correlate and predict the excess molar volumes of the mixtures.



















Similar content being viewed by others
References
Huber, G.W., Iborra, S., Corma, A.: Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering. Chem. Rev. 106, 4044–4098 (2009)
Auger, E., Coquelet, C., Valtz, A., Nala, M., Naidoo, P., Ramjugernath, D.: Equilibrium data and GC-PC SAFT predictions for furanic extraction. Fluid Phase Equilib. 430, 57–66 (2016)
Gladstone, J.H.: Refraction-equivalents of organic compounds. J. Chem. Soc. 45, 241–259 (1884)
Redlich, O., Kister, A.: Algebraic representation of thermodynamic properties and the classification of solutions. Ind. Eng. Chem. 40, 345–348 (1948)
Desnoyers, J., Perron, G.: Treatment of excess thermodynamic quantities for liquid mixtures. J. Solution Chem. 26, 749–755 (1997)
Rowley, R.L.: DIPPR® Data Compilation of Pure Chemical Properties. Design Institute for Physical Properties (2010)
Sharma, N., Kumar, D.: Study and design of eugenol derivatives as potent antioxidant using quantum mechanical method. Int. J. Appl. Pharm. Biol. Res. 1, 24–32 (2016)
Patterson, D., Delmas, G.: Corresponding states theories and liquid models. Discuss. Faraday Soc. 49, 98–105 (1970)
Gepert, M., Zorębski, E., Leszczyńska, A.: Is Flory’s model the best tool for studying the thermodynamic properties of any kind of binary mixtures? Fluid Phase Equilib. 233, 157–169 (2005)
Galvao, A.C., Francesconi, A.Z.: Application of the Prigogine–Flory–Patterson model to excess molar enthalpy of binary liquid mixtures containing acetonitrile and 1-alkanol. J. Mol. Liquids 107, 127–139 (2003)
Torres, R.B., Pina, C.G., Francesconi, A.Z.: Application of the Prigogine–Flory–Patterson theory to excess molar volume of binary mixtures of acetonitrile with 1-alkanols. J. Mol. Liq. 139, 110–116 (2008)
Piñeiro, Á., Amigo, A., Bravo, R., Brocos, P.: Re-examination and symmetrization of the adjustable parameters of the ERAS model. Fluid Phase Equilib. 173, 211–239 (2000)
Flory, P.J.: Statistical thermodynamics of liquid mixtures. J. Am. Chem. Soc. 87, 1833–1838 (1965)
Valtz, A., Coquelet, C., Boukais-Belaribi, G., Dahmani, A., Belaribi, F.B.: Volumetric properties of binary mixtures of 1,2-dichloroethane with polyethers from (283.15 to 333.15) K and at atmospheric pressure. J. Chem. Eng. Data 56, 1629–1657 (2011)
Iloukhani, H., Rezaei-Sameti, M.: Volumetric properties of methylcyclohexane with n-alkanes (C5–C10) at 293.15, 298.15 and 303.15 K. Comparison with Prigogine–Flory–Patterson theory. J. Mol. Liq. 126, 62–68 (2006)
Lemmon, E.W., Huber, M.L., McLinden, M.O.: NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties-REFPROP, Version 10, National Institute of Standards and Technology (2013)
Guthrie Jr., G.B., Scott, D.W., Hubbard, W.N., Katz, C., McCullough, J.P., Gross, M.E., Williamson, K.D., Waddington, G.: Thermodynamic properties of furan. J. Am. Chem. Soc. 74, 4662–4669 (1952)
Karabaev, M.K.: Kinetische, thermische und kalorische Eigenschaften von fluessigen Eugenol. Izv. Akad. Nauk Uzb. SSR Ser. Fiz. Mat. Nauk, pp. 72–74 (1983)
Mel’nikov, G.A., Vervenko, V.N., Otpuschennikov, N.F.: Complex study of the elastic and thermal properties of hydrocarbons and their halogen derivatives by the acoustic method. Zh. Fiz. Khim. 62, 798 (1988). in Russian
Rubini, K., Francesconi, R., Bigi, A., Comelli, F.: Excess molar enthalpies and heat capacities of dimethyl sulfoxide + seven normal alkanols at 303.15 K and atmospheric pressure. Thermochim. Acta 452, 124–127 (2007)
Hansen, C.M.: Hansen Solubility Parameters: A User’s Handbook. CRC Press, Boca Raton (2002)
Mulder, M.H.V., Smolders, C.A.: On the mechanisms of separation of ethanol/water mixtures by pervaporation. I. Calculations of concentration profiles. J. Membr. Sci. 17, 289–307 (1984)
Acknowledgments
Financial support from the ANR of France through the project Memobiol (ANR-09-CP2D-10-04 MEMOBIOL) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Coquelet, C., Auger, E. & Valtz, A. Density and Excess Volume for Four Systems Involving Eugenol and Furan. J Solution Chem 48, 455–488 (2019). https://doi.org/10.1007/s10953-019-00870-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-019-00870-6