Abstract
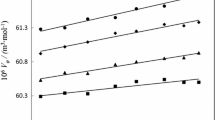
Novel ionic liquids containing the domiphen cation, as an active pharmaceutical ingredient ionic liquid (API-IL), domiphen l-proline ([DOM][l-PRO]) was synthesized and the interactions of domiphen l-proline with some small biomolecules (amino acids and glycyl dipeptides) were investigated at different temperatures from density and UV–Vis spectroscopy measurements. The apparent molar volumes, standard partial molar volumes, transfer volumes, hydration numbers and partial molar expansibilities of small biomolecules have been calculated. Group contributions of amino acids/dipeptides to the standard partial molar volume were determined and the contributions from the zwitterionic end group (NH3 +, COO−), CH2 and (CH2CONH) groups have been obtained. The binding constants between small biomolecules and [DOM][l-PRO] were also obtained. A detailed insight into the physicochemical interactions in the ternary systems was obtained through the perusal of these parameters. Compared with [DOM][Br], [DOM][l-PRO] interacts with small biomolecules weakly.





Similar content being viewed by others
References
Rogers, R.D., Seddon, K.R.: Ionic liquids solvents of the future. Science 302, 792–793 (2003)
Welton, T.: Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 99, 2071–2084 (1999)
Pinto, P.C.A.G., Ribeiro, D.M.G.P., Azevedo, A.M.O., Justina, V.D., Cunha, E., Bica, K., Vasiloiu, M., Reisa, S., Lucia, M., Saraiva, M.F.S.: Active pharmaceutical ingredients based on salicylate ionic liquids: insights into the evaluation of pharmaceutical profiles. New J. Chem. 37, 4095–4102 (2013)
Hough, W.L., Smiglak, M., Rodriguez, H., Swatloski, R.P., Spear, S.K., Daly, D.T., Pernak, J., Grisel, J.E., Carliss, R.D., Soutullo, M.D., Davis, J., Rogers, R.D.: The third evolution of ionic liquids: active pharmaceutical ingredients. New J. Chem. 31, 1429–1436 (2007)
Ferraz, R., Branco, L.C., Marrucho, I.M., Araujo, J.M.M., Rebelo, L.P.N., Ponte, M.N., Prudêncio, C., Noronha, J.P., Petrovski, Z.: Development of novel ionic liquids based on ampicillin. Med. Chem. Commun. 3, 494–497 (2012)
Florindo, C., Araujo, J.M.M., Alves, F., Matos, C., Ferraz, R., Prudêncio, C., Noronha, J.P., Petrovski, Z., Branco, L.C., Rebelo, L.P.N., Marrucho, I.M.: Evaluation of solubility and partition properties of ampicillin-based ionic liquids. Int. J. Pharm. 456, 553–559 (2013)
Bica, K., Rijksen, C., Nieuwenhuyzen, M., Rogers, R.D.: In search of pure liquid salt forms of aspirin: ionic liquid approaches with acetylsalicylic acid and salicylic acid. Phys. Chem. Chem. Phys. 12, 2011–2017 (2010)
Hough-Troutman, W.L., Smiglaki, M., Griffin, S., Reichert, W.M., Mirska, I., Jodynis-Liebert, J., Adamska, T., Nawrot, J., Stasiewicz, M., Rogers, R.D., Pernak, J.: Ionic liquids with dual biological function: sweet and anti-microbial, hydrophobic quaternary ammonium-based salts. New J. Chem. 33, 26–33 (2009)
Cybulski, J., Wisniewska, A., Kulig-Adamiak, A., Dabrowski, Z., Praczyk, T., Michalczyk, A., Walkiewicz, F., Materna, K., Pernak, J.: Mandelate and prolinate ionic liquids: synthesis, characterization, catalytic and biological activity. Tetrahedron Lett. 52, 1325–1328 (2011)
Stoimenovski, J., MacFarlane, D.R., Bica, K., Rogers, R.D.: Crystalline vs. ionic liquid salt forms of active pharmaceutical ingredients: a position paper. Pharm. Res. 27, 521–526 (2010)
Alves, F., Oliveira, F.S., Schroder, B., Matos, C., Marrucho, I.M.: Synthesis, characterization, and liposome partition of a novel tetracycline derivative using the ionic liquids framework. J. Pharm. Sci. 102, 1504–1512 (2013)
Shekaari, H., Zafarani-Moattar, M.T., Mirheydari, S.N.: Conductometric analysis of 1-butyl-3-methylimidazolium ibuprofenate as an active pharmaceutical ingredient ionic liquid (API-IL) in the aqueous amino acids solutions. J. Chem. Thermodyn. 103, 165–175 (2016)
Shekaari, H., Zafarani-Moattar, M.T., Mirheydari, S.N.: Thermodynamic properties of 1-butyl-3-methylimidazolium salicylate as an active pharmaceutical ingredient ionic liquid (API-IL) in aqueous solutions of glycine and l-alanine at T = (288.15–318.15) K. Thermochim. Acta 637, 51–68 (2016)
Yan, Z.N., Wen, X.L., Kang, Y.X., Chu, W.W.: Intermolecular interactions of a-amino acids and glycyl dipeptides with the drug domiphen bromide in aqueous solutions analyzed by volumetric and UV–Vis spectroscopy methods. J. Chem. Thermodyn. 101, 300–307 (2016)
Redlich, O., Meyer, D.M.: The molal volumes of electrolytes. Chem. Rev. 64, 221–227 (1964)
Belibagli, K., Ayranci, E.: Viscosities and apparent molar volumes of some amino acids in water and in 6 M guanidine hydrochloride at 25 °C. J. Solution Chem. 19, 867–882 (1990)
Yan, Z.N., Wang, X.L., Bai, X.R., Wang, S.Q., Wang, J.J.: Volumetric, conductometric and fluorescence probe studies of interactions between glycyl dipeptides and sodium caprylate in aqueous media. J. Chem. Thermodyn. 52, 89–94 (2012)
Yan, Z.N., Liu, R.L., Wu, S.Y., Bai, X.R., Wang, J.J.: Effect of temperature on the interactions of glycyl dipeptides with sodium perfluorooctanoate in aqueous solution: volumetric, conductometric, and spectroscopic study. J. Chem. Thermodyn. 57, 360–366 (2013)
Yan, Z.N., Wu, S.Y., Pan, Q., Gen, R., Gu, B.X., Wang, J.J.: Interactions of dipeptides with Triton X-100 in aqueous solution: a volumetric and spectroscopic study. J. Chem. Thermodyn. 71, 112–117 (2014)
Rajagopal, K., Gladson, S.E.: Partial molar volume and partial molar compressibility of four homologous α-amino acids in aqueous sodium fluoride solutions at different temperatures. J. Chem. Thermodyn. 43, 852–867 (2011)
Anwar, A., Vidiksha, B., Priyanka, B.: Volumetric study of α-amino acids and their group contributions in aqueous solutions of cetyltrimethylammonium bromide at different temperatures. J. Mol. Liq. 177, 209–214 (2013)
Chauhan, S., Pathania, L., Sharma, K., Kumar, G.: Volumetric, acoustical and viscometric behavior of glycine and Dl-alanine in aqueous furosemide solutions at different temperatures. J. Mol. Liq. 212, 656–664 (2015)
Wen, X.L., Yan, Z.N., Kang, Y.X., Zhang, S.Y.: Apparent molar volume, conductivity, and fluorescence studies of ternary systems of dipeptides plus ionic liquids ([C(n)mim]Br, n = 10, 14) + water at different temperatures. Colloid Polym. Sci. 293, 2485–2495 (2015)
Yan, Z.N., Wang, J.J., Liu, W.B., Lu, J.S.: Apparent molar volumes and viscosity B-coefficients of some amino acids in aqueous solutions from 278.15 to 308.15 K. Thermochim. Acta 334, 17–27 (1999)
Pal, A., Chauhan, N.: Volumetric behaviour of amino acids and their group contributions in aqueous lactose solutions at different temperatures. J. Chem. Thermodyn. 43, 140–146 (2011)
Mishra, A.K., Ahluwalia, J.C.: Apparent molal volumes of amino acids, N-acetylamino acids, and peptides in aqueous solutions. J. Phys. Chem. 88, 86–92 (1984)
Iqbal, M., Chaudhary, M.A.: Effect of temperature on volumetric and viscometric properties of some non-steroidal anti-inflammatory drugs in aprotic solvents. J. Chem. Thermodyn. 42, 951–956 (2010)
Singh, S.K., Kundu, A., Kishore, N.: Interactions of some amino acids and glycine peptides with aqueous sodium dodecyl sulfate and cetyltrimethylammonium bromide at T = 298.15 K: a volumetric approach. J. Chem. Thermodyn. 36, 7–16 (2004)
Franks, F., Quickenden, M.A., Reid, D.S., Watson, B.: Calorimetric and volumetric studies of dilute aqueous of cycle ether derivatives. Trans. Faraday Soc. 66, 582–589 (1970)
Yan, Z.N., Sun, X.M., Li, W.W., Li, Y., Wang, J.J.: Interactions of glutamine dipeptides with sodium dodecyl sulfate in aqueous solution measured by volume, conductivity, and fluorescence spectra. J. Chem. Thermodyn. 43, 1468–1474 (2011)
Berlin, E., Pallansch, M.J.: Densities of several proteins and l-amino acids in the dry state. J. Phys. Chem. 72, 1887–1889 (1968)
Yan, Z.N., Zhao, Y., Xing, R.H., Wang, X.G., Wang, J.J.: Volumetric and conductometric behavior at T = 298.15 K of 2-[(2-aminoacetyl)amino]acetic acid, 2-[(2-aminoacetyl)amino]-3-methylbutanoic acid, and (2S)-2-[(2-aminoacetyl)amino]-4-methylpentanoic acid with sodium hexanoate. J. Chem. Eng. Data 55, 759–764 (2010)
Sinha, B., Sarkar, A., Roy, P., Brahman, D.: Physicochemical properties of l-alanine in aqueous silver sulphate solutions at (298.15, 308.15, and 318.15) K. Int. J. Thermophys. 32, 2062–2078 (2011)
Hepler, L.: Thermal expansion and structure in water and aqueous solutions. Can. J. Chem. 47, 4613–4617 (1969)
Yazdanbakhsh, M.R., Mohammadi, A., Mohajerani, E., Nemati, H., Nataj, N.H., Moheghi, A., Naeemikhah, E.: Novel azo disperse dyes derived from N-benzyl-N-ethyl-aniline: synthesis, solvatochromic and optical properties. J. Mol. Liq. 151, 107–112 (2010)
Kumar, H., Kaur, K.: Interaction of antibacterial drug ampicillin with glycine and its dipeptides analyzed by volumetric and acoustic methods. Thermochim. Acta 551, 40–45 (2013)
Scott, R.L.: Some comments on the Benesi–Hildebrand equation. Rec. Trav. Chim. Pays-Bas 75, 787–789 (1956)
Acknowledgements
The project was financially supported by the Natural Science Foundation of China (No. 21573199).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yan, Z., Chu, W., Shen, S. et al. Volumetric and UV Absorption Studies on Interactions of an Active Pharmaceutical Ingredient Ionic Liquid (API-IL) Domiphen l-Proline with Amino Acids and Glycyl Dipeptides in Aqueous Solution at T = (293.15–308.15) K. J Solution Chem 46, 1658–1679 (2017). https://doi.org/10.1007/s10953-017-0671-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-017-0671-2