Abstract
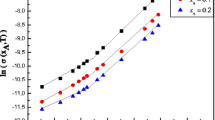
Conductivity measurements were performed for aqueous mixtures of sodium polystyrene sulfonate (NaPSS) and 1-octyl-3-methylimidazolium bromide ([OMIm]Br) at 288.15, 298.15 and 308.15 K. Scaling theory is used for the description of the electrical conductance of the polyelectrolytes. The results indicate that the fraction of uncondensed counterions is decreased by increasing the temperature or concentration of [OMIm]Br. Conductivity measurements for 1-octyl-3-methylimidazolium bromide were performed in aqueous solutions of sodium polystyrene sulfonate. Data analysis was performed using the Quint–Viallard conductivity equation and the low concentration chemical model. Limiting molar conductivities of [OMIm]Br (Λ 0) and the association constant (K A) were determined. The molar conductivity of [OMIm]Br in aqueous solutions of NaPSS increased with increasing temperature. Values of activation energy for viscous flow are higher than the values of activation enthalpy of charge transport; therefore, it can be concluded that, in addition to ion transfer, the formation and breaking of hydrogen bonds is responsible for a portion of the charge transfer. The results of UV–Vis spectroscopic and quantum chemical calculations confirmed the existence of hydrogen bonding between [OMIm]+ and [PSS]–.






Similar content being viewed by others
References
Wang, P., Anderko, A.: Modeling chemical equilibria, phase behavior, and transport properties in ionic liquid systems. Fluid Phase Equilib. 302, 74–82 (2010)
Bahadur, I., Momin, M.I.K., Koorbanally, N.A., Sattari, M., Ebenso, E.E., Katata-Seru, L.M., Singh, S., Ramjugernath, D.: Interactions of polyvinylpyrrolidone with imidazolium based ionic liquids: spectroscopic and density functional theory studies. J. Mol. Liq. 213, 13–16 (2016)
Döker, M., Gmehling, J.: Measurement and prediction of vapor–liquid equilibria of ternary systems containing ionic liquids. Fluid Phase Equilib. 227, 255–266 (2005)
Kato, R., Krummen, M., Gmehling, J.: Measurement and correlation of vapor–liquid equilibria and excess enthalpies of binary systems containing ionic liquids and hydrocarbons. Fluid Phase Equilib. 224, 47–54 (2004)
Santiago, R.S., Santos, G.R., Aznar, M.: UNIQUAC correlation of liquid–liquid equilibrium in systems involving ionic liquids: the DFT-PCM approach. Part II. Fluid Phase Equilib. 293, 66–72 (2010)
Bešter-Rogač, M., Hunger, J., Stoppa, A., Buchner, R.: 1-Ethyl-3-methylimidazolium ethylsulfate in water, acetonitrile, and dichloromethane: molar conductivities and association constants. J. Chem. Eng. Data 56, 1261–1267 (2011)
Bešter-Rogač, M., Stoppa, A., Hunger, J., Hefter, G., Buchner, R.: Association of ionic liquids in solution: a combined dielectric and conductivity study of [bmim][Cl] in water and in acetonitrile. Phys. Chem. Chem. Phys. 13, 17588–17598 (2011)
De Gennes, P.G., Pincus, P., Velasco, R.M., Brochard, F.: Remarks on polyelectrolyte conformation. J. Phys. 37, 1461–1473 (1976)
Barrat, J.L., Joanny, J.F.: Theory of polyelectrolyte solutions. Adv. Chem. Phys. 94, 1–66 (1996)
Dobrynin, A.V., Rubinstein, M.: Theory of polyelectrolytes in solutions and at surfaces. Prog. Polym. Sci. 30, 1049–1118 (2005)
Manning, G.S., Ray, J.: Counterion condensation revisited. J. Biomol. Struct. Dyn. 16, 461–476 (1998)
Pal, A., Yadav, S.: Binding interaction between 1-octyl-3-methylimidazolium bromide and sodium polystyrene sulfonate in aqueous solution. Fluid Phase Equilib. 412, 71–78 (2016)
Bončina, M., BešterRogač, M.: Global thermodynamic analysis of conductivity data. Acta Chim. Slov. 59, 536–541 (2012)
Sharma, R., Das, C., Dahal, S., Das, B.: Polyion–counterion interactions in sodium carboxymethylcellulose–ethylene glycol–water ternary solutions. Carbohydr. Polym. 92, 1546–1554 (2013)
Barthel, J.M.G., Krienke, H., Kunz, W.: Physical Chemistry of Electrolyte Solutions: Modern Aspects. Springer, Berlin (1998)
Quint, J., Viallard, A.: Electrical conductance of electrolyte mixtures of any type. J. Solution Chem. 7, 533–548 (1978)
Pei, Y., Wang, J., Liu, L., Wu, K., Zhao, Y.: Liquid–liquid equilibria of aqueous biphasic systems containing selected imidazolium ionic liquids and salts. J. Chem. Eng. Data 52, 2026–2031 (2007)
Yang, J.-Z., Tong, J., Li, J.-B.: Study of the volumetric properties of the aqueous ionic liquid 1-methyl-3-pentylimidazolium tetrafluoroborate. J. Solution Chem. 36, 573–582 (2007)
Mehrdad, A., Shekaari, H., Niknam, Z.: Viscometric studies of interactions between ionic liquid 1-octyl-3-methyl-imidazolium bromide and polyvinyl pyrrolidone in aqueous solutions. J. Chem. Thermodyn. 79, 1–7 (2014)
Yu, M., Li, S.M., Li, X.Y., Zhang, B.J., Wang, J.J.: Acute effects of 1-octyl-3-methylimidazolium bromide ionic liquid on the antioxidant enzyme system of mouse liver. Ecotoxicol. Environ. Saf. 71, 903–908 (2008)
Stark, A., Ott, D., Kralisch, D., Kreisel, G., Ondruschka, B.: Ionic liquids and green chemistry: a lab experiment. J. Chem. Educ. 87, 196–201 (2010)
Mou, Z., Li, P., Bu, Y., Wang, W., Shi, J., Song, R.: Investigations of coupling characters in ionic liquids formed between the 1-ethyl-3-methylimidazolium cation and the glycine anion. J. Phys. Chem. B 112, 5088–5097 (2008)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery, J.A., Vreven, T., Kudin, K.N., Burant, J.C., Millam, J.M., Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G.A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J.E., Hratchian, H.P., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Ayala, P.Y., Morokuma, K., Voth, G.A., Salvador, P., Dannenberg, J.J., Zakrzewski, V.G., Dapprich, S., Daniels, A.D., Strain, M.C., Farkas, O., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Ortiz, J.V., Cui, Q., Baboul, A.G., Clifford, S., Cioslowski, J., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R.L., Fox, D.J., Keith, T., Laham, A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Gonzalez, C., Pople, J.A.: Gaussian 03. Revision B.03. Gaussian, Inc., Pittsburgh (2003)
Becke, A.D.: Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993)
Lee, C., Yang, W., Parr, R.G.: Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988)
Bulat, F.A., Toro-Labbé, A., Brinck, T., Murray, J.S., Politzer, P.: Quantitative analysis of molecular surfaces: areas, volumes, electrostatic potentials and average local ionization energies. J. Mol. Model. 16, 1679–1691 (2010)
Biegler Konig, F.W., Schonbohm, J., Bayles, D.: Software news and updates AIM2000. J. Comput. Chem. 22, 545–559 (2001)
Glendening, E.D., Reed, A.E., Carpenter, J.E., Weinhold, F.: NBO 3.0 program manual. Gaussian Inc, Pittsburgh (1995)
Manning, G.S.: Limiting laws and counterion condensation in polyelectrolyte solutions I. Colligative properties. J. Chem. Phys. 51, 924–933 (1969)
Manning, G.S.: Limiting law for the conductance of the rod model of a salt-free polyelectrolyte solution. J. Phys. Chem. 79, 262–265 (1975)
Manning, G.S.: The critical onset of counterion condensation: A survey of its experimental and theoretical basis. Ber. Bunsenges. Phys. Chem. 100, 909–922 (1996)
Bhattarai, A., Nandi, P., Das, B.: The effects of concentration, relative permittivity and temperature on the transport properties of sodium polystyrenesulphonate in methanol–water mixed solvent media. J. Polym. Res. 13, 475–482 (2006)
Ghosh, D., Bhattarai, A., Das, B.: Electrical conductivity of sodium polystyrenesulfonate in acetonitrile–water-mixed solvent media: experiment and data analysis using the Manning counterion condensation model and the scaling theory approach. Colloid Polym. Sci. 287, 1005–1011 (2009)
Colby, R.H., Boris, D.C., Krause, W.E., Tan, J.S.: Polyelectrolyte conductivity. J. Polym. Sci. B. Polym. Phys. 35, 2951–2960 (1997)
Bešter-Rogač, M., Hunger, J., Stoppa, A., Buchner, R.: Molar conductivities and association constants of 1-butyl-3-methylimidazolium chloride and 1-butyl-3-methylimidazolium tetrafluoroborate in methanol and DMSO. J. Chem. Eng. Data 55, 1799–1803 (2009)
Bončina, M., Apelblat, A., Barthel, J., Bešter-Rogač, M.: Investigation of the dissociation and dimerization of cyclamic acid in aqueous solutions by means of a conductometric method. J. Solution Chem. 37, 1561–1574 (2008)
Bešter-Rogač, M., Stoppa, A., Buchner, R.: Ion association of imidazolium ionic liquids in acetonitrile. J. Phys. Chem. B 118, 1426–1435 (2014)
Shannon, R.D.: Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 32, 751–767 (1976)
Apelblat, A.: Representation of electrical conductances for polyvalent electrolytes by the Quint-Viallard conductivity equation. Part 4. Symmetrical 2:2, 3:3 and unsymmetrical 2:1, 3:1 and 1:3 type electrolytes in pure organic solvents. J. Solution Chem. 40, 1234–1257 (2011)
Apelblat, A., Neueder, R., Barthel, J.: Electrolyte data collection: Electrolyte conductivities, ionic conductivities and dissociation constants of aqueous solutions of organic monobasic acids: C8H5NO2–C14H12O3. Dechema 1, 503 (2005)
Malmberg, C.G., Maryott, A.A.: Dielectric constant of water from 0° to 100°. C. J. Res. Nat. Bureau Stand. 56, 1–8 (1956)
Skekaari, H., Kazempour, A.: Effect of ionic liquid, 1-octyl-3-methylimidazolium bromide on the thermophysical properties of aqueous d-glucose solutions at 298.15 K. Fluid Phase Equilib. 309, 1–7 (2011)
Skekaari, H., Zafarani-Moattar, M.T., Ghaffari, F.: Volumetric, acoustic and conductometric studies of acetaminophen in aqueous ionic liquid, 1-octyl-3-methylimidazolium bromide at T = 293.15–308.15 K. Phys. Chem. Res. 4, 119–141 (2016)
Shaw, H.R.: Viscosities of magmatic silicate liquids; an empirical method of prediction. Am. J. Sci. 272, 870–893 (1972)
Krupenie, P.H., Benesch, W.: Electronic transition moment Integrals for first ionization of CO and the AX transition in CO+. Some limitations on the use of the r-centroid approximation. J. Res. Natl. Bur. Stand. A 72A, 495–503 (1968)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mehrdad, A., Parvini, E. Interactions of Sodium Polystyrene Sulfonate with 1-Octyl-3-methylimidazolium Bromide in Aqueous Solution: Conductometric, Spectroscopic and Density Functional Theory Studies. J Solution Chem 46, 908–930 (2017). https://doi.org/10.1007/s10953-017-0608-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-017-0608-9