Abstract
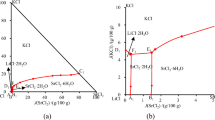
Based on the requirement for the comprehensive exploitation and utilization of the salt lake resources magnesium chloride and potassium chloride, a new technology to produce KCl and ammonium carnallite (NH4Cl·MgCl2·6H2O) by using NH4Cl as salting-out agent to separate carnallite is proposed. The solubilities of quaternary system KCl–MgCl2–NH4Cl–H2O were measured by the isothermal method at t = 60.00 °C and the corresponding phase diagram was plotted and analyzed. The analysis of this phase diagram shows that there are seven saturation points and eight regions of crystallization. These eight regions of crystallization represent salts corresponding to KCl, NH4Cl, MgCl2·6H2O, (K1−n (NH4) n )Cl, ((NH4) n K1−n )Cl, (K1−n (NH4) n )Cl·MgCl2·6H2O, KCl·MgCl2·6H2O and NH4Cl·MgCl2·6H2O. According to the phase diagram analysis and calculations, ammonium carnallite (NH4Cl·MgCl2·6H2O) and KCl can be obtained using carnallite as raw materials and ammonium chloride as salting-out agent at t = 60.00 °C. The new technology shows the advantages of being easy to operate and having low energy consumption. The research on this quaternary phase diagram is the foundation for reasonable development of carnallite resources and comprehensive utilization of the salt lake brines.





Similar content being viewed by others
References
Liu, J.-L., Zhang, Y.-K.: Preparation techniques and development trend of anhydrous magnesium chloride. J. Inorg. Chem. Ind. 39, 10–12 (2007)
Zhou, H., Yuan, J.-J.: Progress in preparation technology of high purity anhydrous magnesium chloride. J. Process Eng. 4, 276–281 (2004)
Liang, B., Liang, Y.-T., Lu, H.-M.: Comprehensive development and utilization of Qinghai salt lake resources and dynamic equilibrium. J. Min. Metall. 10, 65–70 (2001)
Zhang, J.-W.: Discussion Chaerhan salt lake resources comprehensive utilization. Qinghai Sci. Technol. 4, 123–124 (2009)
Zhou, Y., Li, L.J., Wu, Z.J., Li, X.: Exploitation and comprehensive utilization for Qinghai salt lakes. Prog. Chem. Beijing 25, 1613–1624 (2013)
Zheng, M.-P., Xiang, R.-J., Ge, Z.-H.: The sustainable development of potassium, magnesium, lithium, boron mineral resources in our country. Land Resour. Inf. 3, 27–32 (2004)
Zhang, Z.-X., Zeng, Y., Yu, X.-D.: Stable phase equilibrium in aqueous ternary system MgCl2 + NH4Cl + H2O at 298.15 K. J. Chem. Eng. 40, 38–42 (2012)
Biltz, W., Marcus, E.: Über Ammoniumcarnallit. Z. Anorg. Chem. 71, 166–181 (1911)
Dou, S.-Y., Zhao, B., Cao, J.-L.: Phase diagrams of the quaternary Mg2+, NH4 +//Cl−, SO 2–4 H2O system at 25 °C and their application. Fluid Phase Equilib. 385, 54–61 (2015)
Dou, S.Y., Zhao, B., Li, L., Xue, C.Y., Gong, X.M.: Cao, J.L: Phase diagram of Mg2+, NH4 +//Cl−, SO 2–4 H2O system at 0 °C and their application. Fluid Phase Equilib. 409, 264–270 (2016)
Dou, S.Y., Guo, H.F., Zhao, B., Xue, C.Y., Cao, J.L.: Phase diagrams of the quaternary system NaCl–MgCl2–NH4Cl in water at 0 and 25 °C and their Application. J. Chem. Eng. Data 61, 450–457 (2016)
Gong, C.Z., Wang, S.Z., Ding, L., Li, G.M., Xia, J.L.: Improvement on main content determ ination method in industrial magnesium sulfate standard. J. Inorg. Chem. Ind. 41, 61–62 (2009)
Department of Chemistry, Zhejiang University. Analytical Chemistry Experiment. Higher Educ. Press: Beijing (2003)
Li, J.-T., Cui, Q., Li, F.-M.: Comparison of determination method of sulfate radical in Glauber salt production. Ch. Chlor-Alkali 7, 33–35 (2008)
Li, Y.-G.: Metallic Solvent Extraction Thermodynamic. Tsinghua University Press, Beijing (1988) Chap. 1
Yang, S.: Determination of chlorion in water by silver measuring method. S Electr. Power. Technol. 15, 311–316 (1996)
Funding
The financial support was received from the National Natural Science Foundation of China (No. 21576066).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, JX., Zhang, GC., Zhao, B. et al. Phase Diagram of the Quaternary System KCl–MgCl2–NH4Cl–H2O at t = 60.00 °C and Their Application. J Solution Chem 46, 58–69 (2017). https://doi.org/10.1007/s10953-016-0558-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-016-0558-7