Abstract
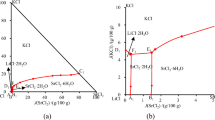
To fully understand the metallogenic regularity and the geochemical equilibrium rules of brine migration and transformation among potassium, barium and strontium in gas field water, the isothermal dissolution equilibrium method was used to study the phase equilibria of quaternary system KCl + BaCl2 + SrCl2 + H2O and its two ternary systems BaCl2 + SrCl2 + H2O and KCl + BaCl2 + H2O at 323 K. The equilibrium liquid phase compositions of each system was measured, the corresponding equilibrium solid phases were identified by chemical analysis and X-ray powder diffraction, and the equilibrium phase diagrams were drawn. No double salts or solid solutions were found in the ternary systems BaCl2 + SrCl2 + H2O and KCl + BaCl2 + H2O at 323 K and the phase diagrams both contain one invariant point, two isothermal solubility curves and two crystallization regions. The hydrate forms in the quaternary system KCl + BaCl2 + SrCl2 + H2O at 323 K are the same as those of its subsystems. The quaternary phase diagram contains one invariant point, three isothermal solubility curves and three crystallization regions, where the equilibrium solid phases are KCl, BaCl2·2H2O and SCl2·6H2O. These results provide basic thermodynamic data for the design of a comprehensive utilization process of potassium, barium and strontium resources in gas field water.








Similar content being viewed by others
References
Tang, P., Liu, B., Xie, W., Wang, P., He, Q., Bao, J., Zhang, Y., Zhang, Z., Li, J., Ma, J.: Synergistic mechanism of combined ferrate and ultrafiltration process for shale gas wastewater treatment. J. Membr. Sci. 641, 119921 (2022)
Vidic, R.D., Brantley, S.L., Vandenbossche, J.M., Yoxtheimer, D., Abad, J.D.: Impact of shale gas development on regional water quality. Science 340, 6134 (2013)
Ni, Y.Y., Zou, C.N., Cui, H.Y., Li, J.L., Nancy, E., Harkness, J.S., Kondash, A.J., Coyte, R.M., Dwyer, G.S., Liu, D., Dong, D.Z., Liao, F.R., Vengosh, A.: The origin of flowback and produced waters from Sichuan Basin, China. Environ. Sci. Technol. 52, 14519–14527 (2018)
Akob, D.M., Cozzarelli, I.M., Dunlap, D.S.: Organic and inorganic composition and microbiology of produced waters from Pennsylvania shale gas wells. Appl. Geochem. 60, 116–125 (2015)
Vengosh, A., Jackson, R.B., Warner, N.: A critical review of the risks to water resources from unconventional shale gas development and hydraulic fracturing in the United States. Environ. Sci. Technol. 48, 8334–8348 (2014)
Pistorius, C.W.F.T.: Polymorphic and dehydration phase boundaries in the systems BaCl2–H2O and BaCl2–D2O to 40 Kilobars. Z. Phys. Chem. Neue Folge 60, 114–125 (1968)
Qiao, Z.P.: Study on the phase diagram of the LaCl3–BaCl2–H2O at 30 ℃. J. Nanyang Norm. Univ. 3, 46–47 (2004)
Zhou, J.L., Jiren, T.B., Fan, J.M., Li, C.C., Feng, X.S.: Measurement and research on the solubility of BaCl2–NaCl–H2O system at 35 ℃. Inner Mon. Petrochem. Ind. 36, 20–22 (2010)
Cao, D.Q., Jin, Y., Chen, H., Yu, J.G.: Phase equilibria determination and solubility calculation of the quaternary system CaCl2–SrCl2–BaCl2–H2O at 338.15 K. CIESC J. 72, 5028–5039 (2021)
Tenu, R., Counioux, J.J.: Le systitme quinaire H2O–NaCl–CaCl2–SrCl2–BaCl2 exploitation de l’isotherme 65 ℃ en vue de l’extraction des chlorures de baryum et de strontium. Chem. Eng. J. 49, 167–175 (1992)
Reddy, D.C., Ananthaswamy, J.: Thermodynamics activity and osmotic coefficients of the ternary system KCl–BaCl2–H2O at 25, 35, and 45 ℃. J. Chem. Eng. Data 35, 144–147 (1990)
Guendouzi, M.E., Benbiyi, A., Dinane, A., Azougen, R.: Determination of water activities and osmotic and activity coefficients of the system NaCl–BaCl2–H2O at 298.15 K. Calphad 27, 375–381 (2003)
Pitzer, K.S.: Thermodynamics of electrolytes. V. Effects of higher-order electrostatic terms. J. Solution Chem. 4, 249–265 (1975)
Assarsson, G.O., Balder, A.: Equilibria between 18 ℃ and 100 ℃ in the aqueous systems containing Sr2+, Mg2+ and Cl−. J. Phys. Chem. 58, 416–417 (1954)
Assarsson, G.O.: Equilibria in aqueous systems containing Na+, K+, Sr2+ and Cl−. J. Phys. Chem. 57, 207–210 (1953)
Li, D., Meng, Q.F., Meng, L.Z., Fan, X.X.: Solid–liquid equilibria in the NaCl–SrCl2–H2O system at 288.15 K. Russ. J. Phys. Chem. A 90, 368–373 (2016)
Wang, F.Y., Hu, B.: Solubility predication in the systems of NaCl–SrCl2–H2O and KCl–SrCl2–H2O at 25 ℃ using Pitzer ion-interaction model. J. Salt Lake Res. 17, 36–39 (2009)
Zhang, X., Sang, S.H., Zhong, S.Y., Huang, W.Y.: Equilibria in the ternary system SrCl2–KCl–H2O and the quaternary system SrCl2–KCl–NaCl–H2O at 323 K. Russ. J. Phys. Chem. A 89, 2322–2326 (2015)
Li, D.W., Sang, S.H., Cui, R.Z., Wei, C.: Solid–liquid equilibria the ternary systems NaCl–SrCl2–H2O and KCl–SrCl2–H2O at 348 K. J. Chem. Eng. Data 60, 1227–1232 (2015)
Zhao, L.R., Li, X.P., Sang, S.H., Gao, Y.Y., He, C.X.: Measurement and prediction of solid–liquid equilibria in the ternary system (KCl–SrCl2–H2O) at 273 and 308 K. Fluid Phase Equilibr. 531, 1–7 (2021)
Filippov, V.K., Fedorov, Y.A., Charykov, N.A.: Thermodynamics of phase equilibria in the potassium, strontium, sodium, chloride, water (K+, Sr2+/Cl−–H2O, Na+, Sr2+/Cl−–H2O and Na+, K+, Sr2+/Cl−–H2O) system at 25 °C. Z. Obshch. Khim. 60, 492–499 (1990)
Niu, Z.D., Cheng, F.Q.: Phase Diagram of Water Salt System and Its Application. Tianjin University Press, Tianjin (2002)
Fosbøl, P.L., Thomsen, K.E., Stenby, H.: Reverse Schreinemakers method for experimental analysis of mixed-solvent electrolyte systems. J. Solution Chem. 38, 1–14 (2009)
Haynes, W.M.: CRC Handbook of Chemistry and Physics, 97nd edn. CRC Press, Boca Raton, London, New York (2016–2017).
Author information
Authors and Affiliations
Contributions
Y-YG, LC and YT did the experiments and wrote the main manuscript text, S-MW, JH, BS, Y-QL, and WY prepared all the figures and data, all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, YY., Wen, SM., Hu, J. et al. Phase Equilibria of Quaternary System KCl + BaCl2 + SrCl2 + H2O and Its Ternary Systems at 323 K. J Solution Chem (2024). https://doi.org/10.1007/s10953-023-01355-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10953-023-01355-3