Abstract
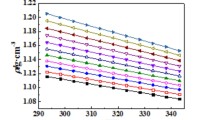
A new ionic liquid, tris(monoethanolamine) citrate (TMEAC), was synthesized and characterized by FT-IR spectrometry and 1H NMR. Densities and viscosities of aqueous TMEAC mixtures were determined at 293.15–323.15 K and atmospheric pressure. The thermal expansion coefficient α, excess molar volume \( V_{\text{m}}^{\text{E}} \), and excess logarithm of viscosity (ln η)E were calculated to evaluate the properties of the aqueous TMEAC ionic liquid. Correlation equations were fitted using these data for the calculation of density and viscosity. The results show that the density increases almost linearly as the mass fraction of TMEAC increases. The mass fraction of TMEAC has little influence on the density at low mass fraction. The viscosity changes significantly and sharply increases at mass fractions larger than 60 %. Both density and viscosity decrease as the temperature increases. The thermal expansion coefficient initially decreases and then increases with increase of the mass fraction of TMEAC. The change of the excess molar volume presents a W-shape. The excess logarithm of viscosity shows a variation where the value initially rises but subsequently drops. Values calculated from the correlation equations are in good agreement with the experimental values. The correlation equations can offer additional results for the aqueous TMEAC ionic liquid.








Similar content being viewed by others
References
Yue, C., Fang, D., Liu, L., Yi, T.-F.: Synthesis and application of task-specific ionic liquids used as catalysts and/or solvents in organic unit reactions. J. Mol. Liq. 163, 99–121 (2011)
Silva, S.S., Duarte, A.R., Carvalho, A.P., Mano, J.F., Reis, R.L.: Green processing of porous chitin structures for biomedical applications combining ionic liquids and supercritical fluid technology. Acta Biomater. 7, 1166–1172 (2011)
Lu, J., Yan, F., John, T.: Advanced applications of ionic liquids in polymer science. Prog. Polym. Sci. 34, 431–448 (2009)
Ma, J., Hong, X.: Application of ionic liquids in organic pollutants control. J. Environ. Manag. 99, 104–109 (2012)
Han, C., Yu, G., Wen, L., Zhao, D., Charles, A., Chen, X.: Data and QSPR study for viscosity of imidazolium-based ionic liquids. Fluid Phase Equilib. 300, 95–104 (2011)
Geng, Y., Wang, T., Yu, D., Peng, C., Liu, H., Hu, Y.: Densities and viscosities of the ionic liquid [C4mim][PF6] + N, N-dimethylformamide binary mixtures at 293.15 K to 318.15 K. Chin. J. Chem. Eng. 2, 256–262 (2008)
Zou, B., Hu, Y., Yu, D., Jiang, L., Liu, W., Song, P.: Functionalized ionic liquid modified mesoporous silica SBA-15: a novel, designable and efficient carrier for porcine pancreas lipase. Colloids Surf. B 88, 93–99 (2011)
Kavitha, T., Attri, P., Venkatesub, P., Rama Devi, R.S., Hofman, T.: Influence of temperature on thermo physical properties of ammonium ionic liquids with N-methyl-2-pyrrolidone. Thermochim. Acta 45, 131–140 (2012)
Baj, S., Chrobok, A., Derfla, S.: A new method for dialkyl peroxides synthesis in ionic liquids as solvents. Green Chem. 8, 292–295 (2006)
Tan, Z.-Q., Liu, J.-F., Pang, L.: Advances in analytical chemistry using the unique properties of ionic liquids. TrAC Trends Anal. Chem. 39, 218–227 (2012)
Attri, P., Venkatesu, P.: Thermodynamic characterization of the biocompatible ionic liquid effects on protein model compounds and their functional groups. Phys. Chem. 13, 6566–6575 (2011)
Blath, J., Christ, M., Deubler, N., Hirth, T., Schiestel, T.: Gas solubilities in room temperature ionic liquids: correlation between RTIL-molar mass and Henry’s law constant. Chem. Eng. J. 172, 167–176 (2011)
Maia, F.M., Rodríguez, O., Macedo, E.A.: Relative hydrophobicity of equilibrium phases in biphasic systems (ionic liquid+water). J. Chem. Thermodyn. 48, 221–228 (2012)
Abbott, A.P., Capper, G., Davies, D.L., Munro, H.L., Rasheed, R.K., Tambyrajah, V.: Preparation of novel, moisture-stable, Lewis-acidic ionic liquids containing quaternary ammonium salts with functional side chains. Chem. Commun. 19, 2010–2011 (2001)
Heintz, A., Wertz, C.: Ionic liquids: a most promising research field in solution chemistry and thermodynamics. Pure Appl. Chem. 78, 1587–1593 (2006)
Soriano, A.N., Doma, B.T.J., Li, M.H.: Carbon dioxide solubility in some ionic liquids at moderate pressures. J. Taiwan Inst. Chem. Eng. 40, 387–393 (2009)
Yuan, X.L., Zhang, S.J., Lu, X.M.: Hydroxyl ammonium ionic liquids: synthesis, properties, and solubility of SO2. J. Chem. Eng. Data 52, 596–599 (2007)
Zhai, L.-Z., Zhong, Q., Wang, X.-R.: Effects of additives on wet flue gas desulfurization with ethylenediamine. J. Fuel Chem. Technol. 38, 242–246 (2010)
Ochedzan-Siodlak, W., Dziubek, K., Siodlak, D.: Densities and viscosities of imidazolium and pyridinium chloroaluminate ionic liquids. J. Mol. Liq. 177, 85–93 (2013)
Domanska, U., Krolikowska, M.: Density and viscosity of binary mixtures of thiocyanate ionic liquids+water as a function of temperature. J. Solution Chem. 41, 1422–1445 (2012)
Kurnia, K.A., Ariwahjoedi, B., Abdul Mutalib, M.I., Murugesan, T.: Density and excess molar volume of the protic ionic liquid bis(2-hydroxyethyl)ammonium acetate with alcohols. J. Solution Chem. 40, 470–480 (2011)
Heintz, A., Klasen, D., Lehmann, J.K.: Excess molar volumes and viscosities of binary mixtures of methanol and the ionic liquid 4-methyl-N-butylpyridinium tetrafluoroborate at 25, 40, and 50 °C. J. Solution Chem. 31, 467–476 (2002)
Diogo, J.C.F., Caetano, F.J.P., Fareleira, J.M.N.A., Wakeham, W.A.: Viscosity measurements on ionic liquids: a cautionary tale. Int. J. Thermophys. (2014). doi:10.1007/s10765-013-1487-y
Chandra, A., Patidar, V., Singh, M., Kale, R.K.: Physicochemical and friccohesity study of glycine, l-alanine and l-phenylalanine with aqueous methyltrioctylammonium and cetylpyridinium chloride from T = (293.15 to 308.15) K. J. Chem. Thermodyn. 65, 18–28 (2013)
Arce, A., Rodil, E., Soto, A.: Physical and excess properties for binary mixtures of 1-methyl-3-octylimidazolium tetrafluoroborate, [Omim][BF4], ionic liquid with different alcohols. J. Solution Chem. 35, 63–78 (2006)
Plechkova, N.V., Seddon, K.R.: Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 37, 123–150 (2008)
Andreatta, A.E., Rodil, E., Arce, A., Soto, A.: Surface tension of binary mixtures of 1-alkyl-3-methyl-imidazolium bis(trifluoromethylsulfonyl)imide ionic liquids with alcohols. J. Solution Chem. 43, 404–420 (2014)
Shojaeian, A., Haghtalab, A.: Solubility and density of carbon dioxide in different aqueous alkanolamine solutions blended with 1-butyl-3-methylimidazolium acetate ionic liquid at high pressure. J. Mol. Liq. 187, 218–225 (2013)
Yang, J.-Z., Tong, J., Li, J.-B.: Study of the volumetric properties of the aqueous ionic liquid 1-methyl-3-pentylimidazolium tetrafluoroborate. J. Solution Chem. 36, 573–582 (2007)
Lu, J.-G., Fan, F., Liu, C., Zhang, H., Ji, Y., Chen, M.-D.: Density, viscosity, and surface tension of aqueous solutions of potassium glycinate+piperazine in the range of (288.15 to 323.15) K. J. Chem. Eng. Data 5, 2706–2709 (2011)
Lu, J.-G., Hua, A.-C., Xu, Z.-W., Fan, F., Cheng, L., Lin, F.-Y.: Measurement and prediction of densities, viscosities, and surface tensions for aqueous solutions of potassium citrate. Fluid Phase Equilib. 327, 9–13 (2012)
Kurnia, K.A., Taib, M.M., Abdul Mutalib, M.I., Murugesan, T.: Densities, refractive indices and excess molar volumes for binary mixtures of protic ionic liquids with methanol at T = 293.15 to 313.15 K. J. Mol. Liq. 159, 211–219 (2011)
Deenadayalu, N., Kumar, S., Bhujrajh, P.: Liquid densities and excess molar volumes for (ionic liquids+methanol+water) ternary system at atmospheric pressure and at various temperatures. J. Chem. Thermodyn. 39, 1318–1324 (2007)
Mokhtarani, B., Sharifi, A., Mortaheb, H.R., Mirzaei, M., Mafi, M., Sadeghian, F.: Density and viscosity of pyridinium-based ionic liquids and their binary mixtures with water at several temperatures. J. Chem. Thermodyn. 41, 323–329 (2009)
Shi, D.-Q., Gao, G.-L., Li, D.-Y., Dong, J.-W., Wang, L.-H.: New device for fast measuring surface tension, density and viscosity of liquids. Fluid Phase Equilib. 273, 87–91 (2008)
Pereiro, A.B., Legido, J.L., Rodriguez, A.: Physical properties of ionic liquids based on 1-alkyl-3-methylimidazolium cation and hexafluorophosphate as anion and temperature dependence. J. Chem. Thermodyn. 39, 1168–1175 (2007)
Wang, S., Jacquemin, J., Husson, P., Hardacre, C., Gomes, M.F.C.: Liquid–liquid miscibility and volumetric properties of aqueous solutions of ionic liquids as a function of temperature. J. Chem. Thermodyn. 41, 1206–1214 (2009)
Machanová, K., Troncoso, J., Jacquemin, J., Bendová, M.: Excess molar volumes and excess molar enthalpies in binary systems N-alkyl-triethylammonium bis(trifluoromethylsulfonyl)imide+methanol. Fluid Phase Equilib. 363, 156–166 (2014)
Zhu, A.-L., Wang, J.-J., Liu, R.-X.: A volumetric and viscosity study for the binary mixtures of 1-hexyl-3-methylimidazolium tetrafluoroborate with some molecular solvents. J. Chem. Thermodyn. 43, 796–799 (2011)
Tovar, C.A., Carballo, E., Cerdeirifia, C.A., Legido, J.L., Romani, L.: Effect of temperature on W-shaped excess molar heat capacities and volumetric properties: oxaalkane–nonane systems. Int. J. Thermophys. 18, 761–777 (1997)
Shah, J.K., Brennecke, J.F., Maginn, E.J.: Thermodynamic properties of the ionic liquid 1-n-butyl-3-methylimidazolium hexafluorophosphate from Monte Carlo simulations. Green Chem. 4, 112–118 (2002)
Acknowledgments
This work was funded by the Jiangsu Province University Natural Science Project (Grant No. 12KJB610003), the Fundamental Research Funds for the Central Universities (Grant No. 30920130122007), the Jiangsu Province Students Practice and Innovation (201310300077X), the Students Practice and Innovation of NUIST (201410300128), and the Laboratory Opening Project of NUIST (2014).
Conflict of interest
The authors declare no competing financial interest.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, H., Lu, CT., Lu, JG. et al. Density, Viscosity and Excess Molar Volume of the Aqueous Ionic Liquid Tris(monoethanolamine) Citrate at 293.15–323.15 K. J Solution Chem 43, 2117–2132 (2014). https://doi.org/10.1007/s10953-014-0270-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-014-0270-4