Abstract
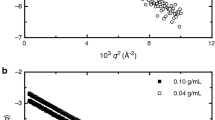
X-ray and neutron scattering have been used to provide insight into the structures of ionic solutions for over a century. Most of the structures discussed in the literature cover distances less than 8 Å. Outside that distance, single scattering bands have, however, been seen. For the non-hydrolyzing salt SrI2 in aqueous solution, a structure sufficient to scatter slow neutrons persists down to a concentration of at least 0.1 mol·L−1 at which the measured average distance between scatterers is over 18 Å. Over the concentration range of 1 to 0.1 mol·L−1, the full distribution of the distances between the scatterers remains within only about 10 Å, the size of an ion and its first hydration shell. This measurably correlated structure of the ions in the solution appears to hold because changes in hydration (and interior distances along any single spatial dimension) require displacements near the size of a water molecule. Together, these facts support a rotatory mechanism for simultaneous ion transport and water countertransport. A formula is presented for calculating the average, equally spaced interscatterer distance as a function of concentration. Using this relationship, the experimental results are interpreted as showing increasing ion association as the salt concentration is raised.




Similar content being viewed by others
References
Prins, J.A.: Zur Beugung von Röntgenstrahlen in Flüssigkeiten un Ionenlösungen. Z. Phys. 71, 445–449 (1931)
Hewlett, C.W.: An experimental study of the scattering of approximately homogeneous X-rays by powdered crystalline carbon, metallic lithium, and liquid benzene, mesitylene, and octane. Phys. Rev. 20, 688–708 (1922)
Ghosh, J.C.: The abnormality of strong electrolytes. Part I. Electrical conductivity of aqueous salt solutions. J. Chem. Soc. 113, 449–458 (1918)
Zernike, F., Prins, J.A.: Die Beugung von Röntgenstrahlen in Flüssigkeiten als Effekt der Molekülanordnung. Z. Phys. 41, 184–194 (1927)
Prins, J.A.: Molecular arrangement and X-ray diffraction in ionic solutions. J. Chem. Phys. 3, 72–80 (1935)
Marques, M.A., Marques, M.I.B., Cabaço, M.I., Gaspar, A.M., Marques, M.P.M., Amado, A.M., da Costa, A.M.A.: Evidence of a local order in concentrated aqueous solutions of salts constituted by ions of different valences. X-ray diffraction and Raman spectroscopy experiments. J. Mol. Liq. 134, 142–150 (2007)
Varela, L.M., Carrete, J., García, M., Gallego, L.J., Turmine, M., Rilo, E., Cabeza, O.: Pseudolattice theory of charge transport in ionic solutions: corresponding states law for the electric conductivity. Fluid Phase Equilib. 298, 280–286 (2010)
Brämer, R., Ruland, W.: The limitations of the paracrystalline model of disorder. Makromol. Chem. 177, 3601–3617 (1976)
Matsuoka, H., Tanaka, H., Hashimoto, T., Ise, N.: Elastic scattering from cubic lattice systems with paracrystalline distortion. Phys. Rev. B 36, 1754–1765 (1987)
Matsuoka, H., Tanaka, H., Iizuke, N., Hashimoto, T., Ise, N.: Elastic scattering from cubic lattice systems with paracrystalline distortion. II. Phys. Rev. B 41, 3854–3856 (1990)
Glinka, C.J., Barker, J.G., Hammouda, B., Krueger, S., Moyer, J.J., Orts, W.J.: The 30-meter small angle neutron scattering instruments at the national institute of standards and technology. J. Appl. Crystallogr. 31, 430–445 (1998)
Kline, S.R.: Reduction and analysis of SANS and USANS data using IGOR Pro. J. Appl. Crystallogr. 39, 895–900 (2006)
Moore, P.B.: Small-angle scattering. Information content and error analysis. J. Appl. Crystallogr. 13, 168–175 (1980)
Gray, R.W.: Encyclopedia Polyhedra: Dodecahedron. http://www.rwgrayprojects.com/rbfnotes/polyhed/PolyhedraData/RhombicDodeca/RhombicDodecahedron.pdf (2007). Accessed March 2012
Frank, H.S., Thompson, P.T.: Fluctuations and the limit of validity of the Debye–Hückel Theory. J. Chem. Phys. 31, 1086–1095 (1959)
Panckhurst, M.H., Macaskill, J.B.: Specific interactions and single-ion activity coefficients in mixed electrolyte solutions. J. Solution Chem. 5, 469–482 (1976)
Hefter, G.: When spectroscopy fails: the measurement of ion pairing. Pure Appl. Chem. 78, 1571–1586 (2006)
Jeffery, S., Hoffmann, P.M., Pethica, J.B., Ramanujan, C., Özer, Ö., Oral, A.: Direct measurement of molecular stiffness and damping in confined water layers. Phys. Rev. B 70, 054114-054111–054114-054118 (2004)
Persson, I.: Hydrated metal ions in aqueous solution: how regular are their structures? Pure Appl. Chem. 82, 1901–1917 (2010)
Marcus, Y.: Ionic radii in aqueous solutions. J. Solution Chem. 12, 271–275 (1983)
Adam, G., Gibbs, J.H.: On the temperature dependence of cooperative relaxation properties in glass-forming liquids. J. Chem. Phys. 43, 139–149 (1965)
Doan, T.H., Sangster, J.: Viscosities of concentrated aqueous solutions of some 1:1, 2:1, and 3:1 nitrates at 25°C. J. Chem. Eng. Data 26, 141–144 (1981)
Lencka, M.M., Anderko, A., Sanders, S.J., Young, R.D.: Modeling viscosity of multicomponent electrolyte solutions. Int. J. Thermophys. 19, 367–378 (1998)
Donati, C., Douglas, J.F., Kob, W., Plimpton, S.J., Poole, P.H., Glotzer, S.C.: Stringlike cooperative motion in a supercooled liquid. Phys. Rev. Lett. 80, 2338–2341 (1998)
Matuura, R., Koga, Y.: Self-diffusion of iodide ion and strontium ion in strontium iodide solutions. Bull. Chem. Soc. Jpn. 32, 1143–1148 (1959)
Robinson, R.A., Stokes, R.H.: Electrolyte Solutions, 2nd rev. edn. Butterworths, London (1959), Appendix 8.10
Acknowledgments
Thanks are due for valuable discussions with John Barker, Craig Brown, Jack Douglas, Boualem Hammouda, Joseph Hubbard, Steve Kline, Susan Krueger, Yun Liu, and Dan Neumann. This work benefitted from SASView software, originally developed by the DANSE project under NSF award DMR-0520547.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rubinson, K.A. Small-Angle Neutron Scattering of Aqueous SrI2 Suggests a Mechanism for Ion Transport in Molecular Water. J Solution Chem 43, 453–464 (2014). https://doi.org/10.1007/s10953-014-0148-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-014-0148-5