Abstract
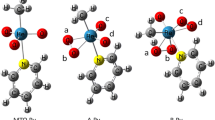
The effect of solvent on the stability and reactivity of methyltrioxorhenium (MTO) for activation of hydrogen peroxide (H2O2) was investigated theoretically. The possible geometries for all Re complexes present in this system, MTO, monoperoxo complexes [A: MeReO2(η 2–O2) and A·H 2 O: MeReO2(η 2–O2)(H2O)], and bisperxo complexes [B: MeReO(η 2–O2)2 and B·H 2 O: MeReO(η 2–O2)2(H2O)] were calculated. Based on the theoretical calculations, species A lacks coordinated water while species B·H 2 O definitely has water coordinated to the Re. The changes of thermodynamic parameters (ΔH and ΔG) for six reactions in the MTO/H2O2, system including formation of mono- and bisperoxo complexes, were determined.






Similar content being viewed by others
References
Herrmann, W.A., Wang, M.: Methyltrioxorhenium as catalyst of a novel aldehyde olefination. Angew. Chem. Int. Ed. Engl. 30, 1641–1643 (1991)
Al-Rawashdeh, N.A.F., Al-Ajlouni, A., Bukallah, S., Bataineh, N.: Activation of H2O2 by methyltrioxorhenium(VII) inside β-cyclodextrin. J. Inclusion Phenom. Macrocycl. Chem. 70, 471–480 (2011)
Ballistreri, F.P., Tomaselli, G.A., Toscano, R.M.: Selective oxidation reactions of diaryl-and dialkyldisulfides to sulfonic acids by CH3ReO3/hydrogen peroxide. Tetrahedron Lett. 50, 6231–6232 (2009)
Di Giuseppe, A., Crucianelli, M., De Angelis, F., Crestini, C., Saladino, R.: Efficient oxidation of thiophene derivatives with homogeneous and heterogeneous MTO/H2O2 systems: a novel approach for oxidative desulfurization (ODS) of diesel fuel. Appl. Catal. B 89, 239–245 (2009)
Espenson, J.H., Pestovsky, O., Huston, P., Staudt, S.: Organometallic catalysis in aqueous solution: oxygen transfer to bromide. J. Am. Chem. Soc. 116, 2869–2877 (1994)
Kühn, F.E., Santos, A.M., Herrmann, W.A.: Organorhenium(VII) and organomolybdenum(VI) oxides: syntheses and application in olefin epoxidation. J. Chem. Soc. Dalton Trans. 7, 2483–2491 (2005)
Kühn, F.E., Scherbaum, A., Herrmann, W.A.: Methyltrioxorhenium and its applications in olefin oxidation, metathesis and aldehyde olefination. J. Organomet. Chem. 689, 4149–4164 (2004)
Michel, T., Betz, D., Cokoja, M., Sieber, V., Kühn, F.E.: Epoxidation of α-pinene catalyzed by methyltrioxorhenium(VII): influence of additives, oxidants and solvents. J. Mol. Catal. A 340, 9–14 (2011)
Owens, G.S., Arias, J., Abu-Omar, M.M.: Rhenium oxo complexes in catalytic oxidations. Catal. Today 55, 317–326 (2000)
Yamazaki, S.: An effective procedure for the synthesis of acid-sensitive epoxides: use of 1-methylimidazole as the additive on methyltrioxorhenium-catalyzed epoxidation of alkenes with hydrogen peroxide. Org. Biomol. Chem. 8, 2377–2385 (2010)
Zhu, Z., Espenson, J.H.: Oxidation of alkynes by hydrogen peroxide catalyzed by methylrhenium trioxide. J. Org. Chem. 60, 7728–7732 (1995)
Bellemin-Laponnaz, S., Le Ny, J.P., Dedieu, A.: Mechanism of the allylic rearrangement of allyloxo metal oxo complexes: an ab initio theoretical investigation. Chem. Eur. J. 5, 57–64 (1999)
Jacob, J., Espenson, J.H., Jensen, J.H., Gordon, M.S.: 1,3-Transposition of allylic alcohols catalyzed by methyltrioxorhenium. Organometallics 17, 1835–1840 (1998)
Owens, G.S., Abu-Omar, M.M.: Methyltrioxorhenium-catalyzed epoxidations in ionic liquids. J. Chem. Soc. Chem. Commun. 1165–1166 (2000)
Lahti, D.W., Espenson, J.H.: Oxidation of sulfoxides by hydrogen peroxide, catalyzed by methyltrioxorhenium(VII). Inorg. Chem. 39, 2164–2167 (2000)
Bernini, R., Mincione, E., Cortese, M., Aliotta, G., Oliva, A., Saladino, R.: A new and efficient Baeyer–Villiger rearrangement of flavanone derivatives by the methyltrioxorhenium/H2O2 catalytic system. Tetrahedron Lett. 42, 5401–5404 (2001)
Phillips, A., Romão, C.: Synthesis of g-butyrolactones by a Baeyer–Villiger oxidation with hydrogen peroxide, catalysed by methyltrioxorhenium. Eur. J. Org. Chem. 1767–1770 (1999)
Eager, M.D., Espenson, J.H.: Activation of molecular oxygen in systems containing methyltrioxorhenium and its derivatives. Inorg. Chem. 38, 2533–2535 (1999)
Adam, W., Mitchell, C.M., Saha-Möller, C.R., Weichold, O.: Host-guest chemistry in a urea matrix: catalytic and selective oxidation of triorganosilanes to the corresponding silanols by methyltrioxorhenium and the urea/hydrogen peroxide adduct. J. Am. Chem. Soc. 121, 2097–2103 (1999)
Stankovic, S., Espenson, J.H.: Oxidation of methyl trimethylsilyl ketene acetals to α-hydroxyesters with urea hydrogen peroxide catalyzed by methyltrioxorhenium. J. Org. Chem. 65, 5528–5530 (2000)
Adam, W., Herrmann, W.A., Lin, J., Saha-Moeller, C.R.: Catalytic oxidation of phenols to p-quinones with the hydrogen peroxide and methyltrioxorhenium(VII) system. J. Org. Chem. 59, 8281–8283 (1994)
Hansen, P.J., Espenson, J.H.: Oxidation of chloride ions by hydrogen peroxide, catalyzed by methylrhenium trioxide. Inorg. Chem. 34, 5839–5844 (1995)
Herrmann, W.A., Fischer, R.W., Marz, D.W.: Methyltrioxorhenium as catalyst for olefin oxidation. Angew. Chem. Int. Ed. Engl. 30, 1638–1641 (1991)
Gonzales, J.M., Distasio Jr, R., Periana, R.A., Goddard III, W.A., Oxgaard, J.: Methylrhenium trioxide revisited: mechanisms for nonredox oxygen insertion in an M–CH3 bond. J. Am. Chem. Soc. 129, 15794–15804 (2007)
Abu-Omar, M.M., Hansen, P.J., Espenson, J.H.: Deactivation of methylrhenium trioxide-peroxide catalysts by diverse and competing pathways. J. Am. Chem. Soc. 118, 4966–4974 (1996)
Al-Ajlouni, A.M., Espenson, J.H.: Kinetics and mechanism of the epoxidation of alkyl-substituted alkenes by hydrogen peroxide, catalyzed by methylrhenium trioxide. J. Org. Chem. 61, 3969–3976 (1996)
Espenson, J.H.: Atom-transfer reactions catalyzed by methyltrioxorhenium (VII)—mechanisms and applications. J. Chem. Soc. Chem. Commun. 479–488 (1999)
Costa, P.J., Calhorda, M.J., Bossert, J., Daniel, C., Romão, C.C.: Photochemistry of methyltrioxorhenium revisited: a DFT/TD-DFT and CASSCF/MS-CASPT2 theoretical study. Organometallics 25, 5235–5241 (2006)
Kuznetsov, M.L., Pombeiro, A.J.L.: Radical formation in the [MeReO3]-catalyzed aqueous peroxidative oxidation of alkanes: a theoretical mechanistic study. Inorg. Chem. 48, 307–318 (2009)
Wu, Y.D., Sun, J.: Transition structures of epoxidation by CH3Re(O)2(O2) and CH3Re(O)(O2)2 and their water adducts. J. Org. Chem. 63, 1752–1753 (1998)
Köstlmeier, S., Häberlen, O.D., Rösch, N., Herrmann, W.A., Solouki, B., Bock, H.: Density functional study on the electronic structure of trioxorhenium organyls. Organometallics 15, 1872–1878 (1996)
Mealli, C., Lopez, J.A., Calhorda, M.J., Romao, C.C., Herrmann, W.A.: Re-C bond homolysis in alkyl-and arylrhenium trioxides: a qualitative MO interpretation. Inorg. Chem. 33, 1139–1143 (1994)
Di Valentin, C., Gandolfi, R., Gisdakis, P., Rösch, N.: Allylic alcohol epoxidation by methyltrioxorhenium: a density functional study on the mechanism and the role of hydrogen bonding. J. Am. Chem. Soc. 123, 2365–2376 (2001)
Gisdakis, P., Rösch, N., Bencze, É., Mink, J., Gonçalves, I.S., Kühn, F.E.: Monomer–dimer equilibria of oxo/imido complexes of heptavalent rhenium: theoretical and spectroscopic investigations. Eur. J. Inorg. Chem. 2001, 981–991 (2001)
Hosseini, F.N., Kamali, K., Nabavizadeh, S.M.: Competition of methyltrioxorhenium (MTO) with osmium tetroxide for pyridines binding: ligand binding assay. Polyhedron 30, 814–820 (2011)
Nabavizadeh, S.M.: Adduct formation of methyltrioxorhenium with mono-and bidentate nitrogen donors: formation constants. Inorg. Chem. 42, 4204–4208 (2003)
Nabavizadeh, S.M.: Thermodynamic studies of the binding of bidentate nitrogen donors with methyltrioxorhenium (MTO) in CHCl3 solution. Dalton Trans. 1644–1648 (2005)
Nabavizadeh, S.M., Akbari, A., Rashidi, M.: Solvent effect on the adduct formation of methyltrioxorhenium (MTO) and pyridine: enthalpy and entropy contributions. J. Chem. Soc. Dalton. Trans. 2423–2427 (2005)
Nabavizadeh, S.M., Rashidi, M.: Lewis acidity of methyltrioxorhenium(VII) (MTO) based on the relative binding strengths of N-donors. J. Am. Chem. Soc. 128, 351–357 (2006)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery Jr, J.A., Vreven, T., Kudin, K.N., Burant, J.C., Millam, J.M., Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G.A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J.E., Hratchian, H.P., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Ayala, P.Y., Morokuma, K., Voth, G.A.S., Dannenberg, J.J., Zakrzewski, V.G., Dapprich, S., Daniels, A.D., Strain, M.C., Farkas, O., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Ortiz, J.V., Cui, Q., Baboul, A.G., Clifford, S., Cioslowski, J., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Gonzalez, C., Pople, J.A.: GAUSSIAN 03, Revision B03. Gaussian Inc., Pittsburgh (2003)
Hay, P.J., Wadt, W.R.: Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 82, 270–283 (1985)
Hariharan, P., Pople, J.: The influence of polarization functions on molecular hydrogenation energies. Theor. Chim. Acta 28, 213–222 (1973)
Ehlers, A., Böhme, M., Dapprich, S., Gobbi, A., Höllwarth, A., Jonas, V., Köhler, K., Stegmann, R., Veldkamp, A., Frenking, G.: A set of f-polarization functions for pseudo-potential basis sets of the transition metals. Chem. Phys. Lett. 208, 111–114 (1993)
Barone, V., Cossi, M.: Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 102, 1995–2001 (1998)
Tomasi, J., Persico, M.: Molecular interactions in solution: an overview of methods based on continuous distributions of the solvent. Chem. Rev. 94, 2027–2094 (1994)
Glendening, E., Reed, A., Carpenter, J., Weinhold, F.: NBO Version 31, as Implemented in Gaussian 03, Revision, D 01. Gaussian Inc., Wallingford (2004)
Herrmann, W.A., Kiprof, P., Rypdal, K., Tremmel, J., Blom, R., Alberto, R., Behm, J., Albach, R.W., Bock, H.: Multiple bonds between main-group elements and transition metals. 86. Methyltrioxorhenium(VII) and trioxo (5-pentamethylcyclopentadienyl) rhenium(VII): structures, spectroscopy and electrochemistry. J. Am. Chem. Soc. 113, 6527–6537 (1991)
Herrmann, W.A., Fischer, R.W., Scherer, W., Rauch, M.U.: Methyltrioxorhenium(VII) as catalyst for epoxidations: structure of the active species and mechanism of catalysis. Angew. Chem. Int. Ed. Engl. 32, 1157–1160 (1993)
Gisdakis, P., Antonczak, S., Köstlmeier, S., Herrmann, W.A., Rösch, N.: Olefin epoxidation by methyltrioxorhenium: a density functional study on energetics and mechanisms. Angew. Chem. Int. Ed. Engl. 37, 2211–2214 (1998)
Acknowledgments
Financial support of the Islamic Azad University, Shiraz Branch, Iran is gratefully acknowledged. We thank Dr. A. Mohajeri for helpful suggestions.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hosseini, F.N., Nabavizadeh, S.M. & Azimi, G. Theoretical Study of the Solvent Effect on the Methyltrioxorhenium/Hydrogen Peroxide System. J Solution Chem 42, 2137–2148 (2013). https://doi.org/10.1007/s10953-013-0101-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-013-0101-z