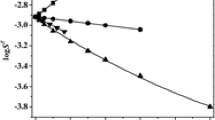
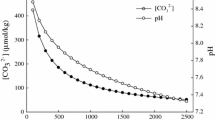
Abstract
Using the “total hydrogen ion concentration scale”, the first, K 1, and second, K 2, stoichiometric dissociation constants of carbonic acid have been determined in synthetic seawater for temperature and salinity ranges of 0–30 °C and 1.5–40, respectively. The values of the K 1 have been determined potentiometrically by means of a cell without liquid junction, composed using a pH-glass electrode and silver–silver chloride electrode. This cell was equilibrated with CO2 at 1 atm total pressure. The same cell was used for the determination of K 2. Dissolved inorganic carbon was measured by the coulometric method, total alkalinity was known from the preparation of the synthetic seawater, and pH measurements provided the data required for the calculations of K 2. Estimated precision is about ±0.003 pK and uncertainty less than 0.01 pK for K 1 and twice that for K 2. Our K 1 data agree with “best” published data within ±0.01 for log10 K 1 in the 30–40 salinity range obtained for natural seawater, but become progressively higher as the salinity decreases. Our results for the second stoichiometric dissociation constants agree within ±0.015 for log10 K 2 in the 30–40 salinity range with available “best” published data for natural seawater, but become progressively lower as the salinity decreases. Since constants obtained in this paper are reliable for the high salinity range (25–40) and strive to the thermodynamic constants at 0 salinity, they are recommended for study of carbonate system in estuaries.





Similar content being viewed by others
References
Sarmiento, J.L., Gruber, N.: Ocean Biogeochemical Dynamics. Princeton University Press, Princeton (2006)
Clegg, S.L., Whitfield, M.: Activity coefficients in natural waters. In: Pitzer, K.S. (ed.) Activity Coefficients in Electrolyte Solutions, 2nd edn, pp. 279–434. CRC Press, Roca Raton (1991)
Millero, F.J., Pierrot, D.: A Chemical equilibrium model for natural waters. Aquat. Geochem. 4, 153–199 (1998)
Dickson, A.G.: Standard potential of the reaction AgCl(s) + 1/2H2(g) = Ag(s) + HCl(aq), and the standard acidity constant of the ion HSO −4 in synthetic seawater from 273.15 to 318.15 K. J. Chem. Thermodyn. 22, 113–127 (1990)
Roy, R.N., Roy, L.N., Vogel, K.M., Porter-Moore, C., Pearson, T., Good, C.E., Millero, F.J., Campbell, D.M.: The dissociation constants of carbonic acid in seawater at salinities 5 to 45 and temperatures 0 to 45 °C. Mar. Chem. 44, 249–267 (1993)
Bradshaw, A.L., Brewer, P.G.: High precision measurements of alkalinity and total carbon dioxide in seawater by potentiometric titration—1. Presence of unknown protolyte(s)? Mar. Chem. 23, 69–86 (1988)
Millero, F.J., Pierrot, D., Lee, K., Wanninkhof, R., Feely, R., Sabine, C.L., Key, R.M., Takahashi, T.: Dissociation constants for carbonic acid determined from field measurements. Deep-Sea Res. I 49, 1705–1723 (2002)
Dickson, A.G., Sabine, C.L., Christian, J.R. (eds.): Guide to Best Practices for Ocean CO2 Measurements. PICES Special Publication (2007)
Dickson, A.G., Millero, F.J.: A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep-Sea Res. 34, 1733–1743 (1987)
Millero, F.J., Byrne, R.H., Wanninkhof, R., Feely, R., Clayton, T., Murphy, P., Lamb, M.F.: The internal consistency of CO2 measurements in the equatorial Pacific. Mar. Chem. 44, 269–280 (1993)
Lee, K., Millero, F.J., Campbell, D.M.: The reliability of the thermodynamic constants for the carbonic acid in seawater. Mar. Chem. 55, 233–245 (1996)
Wanninkhof, R., Lewis, E., Feely, R.A., Millero, F.J.: The optimal carbonate dissociation constants for determining surface water pCO2 from alkalinity and total inorganic carbon. Mar. Chem. 65, 291–301 (1999)
Lee, K., Millero, F.J., Byrne, R.H., Feely, R.A., Wanninkhof, R.: The recommended dissociation constants for carbonic acid in seawater. Geophys. Res. Lett. 27, 229–232 (2000)
Millero, F.J., Graham, T.B., Huang, F., Bustos-Serrano, H., Pierrot, D.: Dissociation constants of carbonic acid in seawater as a function of salinity and temperature. Mar. Chem. 100, 80–94 (2006)
Harned, H.S., Scholes, S.R.: The ionization constants of HCO −3 from 0 to 50°. J. Am. Chem. Soc. 63, 1706–1709 (1941)
Harned, H., Davis, R.: The ionization constant of carbonic acid in water and the solubility of carbon dioxide in water and aqueous salt solutions from 0 to 50°. J. Am. Chem. Soc. 65, 2030–2037 (1943)
Hansson, I.: A new set of acidity constants for carbonic acid and boric acid in seawater. Deep-Sea Res. 20, 461–478 (1973)
Goyet, C., Poisson, A.: New determination of carbonic acid dissociation constants in seawater as function of temperature and salinity. Deep-Sea Res. 36, 1635–1654 (1989)
Mehrbach, C., Culberson, C.H., Hawley, J.E., Pytkowicz, R.M.: Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 18, 897–907 (1973)
Mojica Prieto, F.J., Millero, F.J.: The values of pK 1 + pK 2 for the dissociation of carbonic acid in seawater. Geochim. Cosmochim. Acta 66, 2529–2540 (2002)
Lueker, T.J., Dickson, A.G., Keeling, C.D.: Ocean pCO2 from dissolved inorganic carbon, alkalinity, and equations for K 1 and K 2: validation based on laboratory measurements of CO2 in gas and seawater at equilibrium. Mar. Chem. 70, 105–119 (2000)
McElligott, S., Byrne, R.H., Lee, K., Wanninkhof, R., Millero, F.J., Feely, R.A.: Discrete water column measurements of CO2 fugacity and pHT in seawater: a comparison of direct measurements and thermodynamic calculations. Mar. Chem. 60, 63–73 (1998)
Whitfield, M., Butler, R.A., Covington, A.K.: The determination of pH in estuarine waters. I. Definition of pH scales and the election of buffers. Oceanol. Acta 8, 423–432 (1985)
MacInnes, D.A., Belcher, D.: The thermodynamic ionization constants of carbonic acid. J. Am. Chem. Soc. 55, 2630–2646 (1933)
Johnson, K.M., King, A.E., Sieburth, J.M.: Coulometric TCO2 analysis for marine studies: an introduction. Mar. Chem. 16, 61–82 (1985)
Pitzer, K.S.: Ionic interaction approach: theory and data correlation. In: Pitzer, K.S. (ed.) Activity Coefficients in Electrolyte Solutions, 2nd edn, pp. 75–153. CRC Press, Roca Raton (1991)
De Stefano, C., Foti, C., Gianguzza, A., Sammartano, S.: The interaction of amino acids with the major constituents of natural waters at different ionic strengths. Mar. Chem. 72, 61–76 (2000)
Crea, F., Giacalone, A., Gianguzza, A., Piazzese, D., Sammartano, S.: Modelling of natural and synthetic polyelectrolyte interactions in natural waters by using SIT, Pitzer and ion pairing approaches. Mar. Chem. 99, 93–105 (2006)
Dickson, A.G.: pH scales and proton-transfer reactions in saline media such as sea water. Geochim. Cosmochim. Acta 48, 2299–2308 (1984)
Campbell, D.M., Millero, F.J., Roy, R., Roy, L., Lawson, M., Vogel, K.M., Moore, C.P.: The standard potential for the hydrogen–silver, silver chloride electrode in synthetic seawater. Mar. Chem. 44, 221–233 (1993)
Dickson, A.G., Riley, J.P.: The estimation of acid dissociation constants in seawater media from potentiometric titrations with strong base. I. The ion product of water—K w. Mar. Chem. 7, 89–99 (1979)
Wong, C.S., Tishchenko, P.Ya., Johnson, W.K.: The effects of high CO2 molality on the carbon dioxide equilibrium of seawater. J. Chem. Eng. Data 50, 822–831 (2005)
Weiss, R.F.: Carbon dioxide in water and seawater: the solubility of a non-ideal gas. Mar. Chem. 2, 203–215 (1974)
Wong, C.S., Tishchenko, P.Ya., Johnson, W.K.: Solubility of carbon dioxide in aqueous HCl and NaHCO3 solutions at 278 to 298 K. J. Chem. Eng. Data 50, 817–821 (1974)
Bruevich, S.V.: Instruction for the Performing of Chemical Examination of Seawater. Glavsevmorput’, Moscow (1944)
Talley, L.D., Tishchenko, P.Ya., Luchin, V., Nedashkovskiy, A., Sagalaev, S., Kang, D.-J., Warner, M., Min, D.-H.: Atlas of Japan (East) Sea hydrographic properties in summer, 1999. Prog. Oceanogr. 61, 277–348 (2004)
Bates, R.G.: Determination of pH, Theory and Practice. Wiley, New York (1973)
Pitzer, K.S.: A consideration of Pitzer’s equations for activity and osmotic coefficients in mixed electrolytes. J. Chem. Soc. Faraday Trans. I 80, 3451–3454 (1984)
Tishchenko, P.Ya., Il’ina, E.M., Chichkin, R.V., Wong, C.S.: pH measurements in estuary by means of cell without liquid junction. Okeanologiya 42, 32–41 (2002). (in Russian)
Acknowledgments
This work was partly supported by Grants from the Russian Foundation for Basic Research: 11-05-00241-a, 11-05-98543-r_vostok_a.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tishchenko, P.Y., Wong, C.S. & Johnson, W.K. Measurements of Dissociation Constants of Carbonic Acid in Synthetic Seawater by Means of a Cell Without Liquid Junction. J Solution Chem 42, 2168–2186 (2013). https://doi.org/10.1007/s10953-013-0094-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-013-0094-7