Abstract
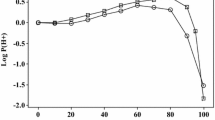
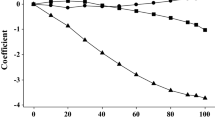
Equations set up for the transfer of neutral solutes from water to water–ethanol mixtures can also be used to correlate the transfer of ions and ionic species, as log10 P, where P is the partition coefficient. Only two additional terms are required in the equations, one for anions and one for cations. The extended equations can fit log10 P values for anions and cations with a standard deviation of about 0.2 to 0.3 log units for transfer of 41 anions and cations from water to various water–ethanol mixtures from 10 % ethanol to 100 % ethanol by volume. The log10 P values for carboxylate anions and protonated amine cations as obtained from the variation of pK a with solvent are quite compatible with log10 P values for simple anions and cations.




Similar content being viewed by others
References
Bates, R.G.: Equilibrium properties of acids and bases in amphiprotic mixed solvents. In: Covington, A.K., Jones, P. (eds.) Hydrogen-Bonded Solvent Systems, pp. 49–86. Taylor and Francis, London (1968)
Hyne, J.B.: The effects of pressure and temperature on reaction rates in aqueous binary solvent systems. In: Covington, A.K., Jones, P. (eds.) Hydrogen-Bonded Solvent Systems, pp. 99–113. Taylor and Francis, London (1968)
Kay, R.L., Cunningham, G.P., Evans, D.F.: The effect of solvent structure on ionic mobilities in aqueous solvent mixtures. In: Covington, A.K., Jones, P. (eds.) Hydrogen-Bonded Solvent Systems, pp. 249–260. Taylor and Francis, London (1968)
Franks, F.: The properties of aqueous solutions of non-electrolytes. In: Franks, F. (ed.) Physico-Chemical Processes in Mixed Aqueous Solvents, pp. 50–70. Heineman, London (1967)
Feakins, D.: Ionic solvation in mixed aqueous solvents. In: Franks, F. (ed.) Physico-Chemical Processes in Mixed Aqueous Solvents, pp. 71–90. Heineman, London (1967)
Blandamer, M.J., Briggs, B., Burgess, J., Elvidge, D., Guardado, P., Hakin, A.W., Radulovic, S., Hubbard, C.D.: Transfer chemical potentials for ions, solubilities of salts and kinetics of reactions involving inorganic complex ions at ambient pressure and 298.2 K in binary aqueous mixtures containing ethanol and propan-2-ol. J. Chem. Soc. Faraday Trans. I 84, 2703–2721 (1988)
Marcus, Y.: Gibbs energies of transfer of anions from water to mixed aqueous organic solvents. Chem. Rev. 107, 3880–3897 (2007)
Abraham, M.H., Acree, W.E. Jr.: Equations for the transfer of neutral molecules and ionic species from water to organic phases. J. Org. Chem. 75, 1006–1015 (2010)
Abraham, M.H., Acree, W.E. Jr.: Solute descriptors for phenoxide anions and their use to establish correlations of rates of reaction of anions with iodomethane. J. Org. Chem. 75, 3021–3026 (2010)
Abraham, M.H., Acree, W.E. Jr.: The transfer of neutral molecules, ions and ionic species from water to ethylene glycol and to propylene carbonate; descriptors for pyridinium cations. New J. Chem. 34, 2298–2305 (2010)
Abraham, M.H., Acree, W.E. Jr.: Partition coefficients and solubilities of compounds in the water–ethanol solvent system. J. Solution Chem. 40(7), 1279–1290 (2011)
Abraham, M.H.: Scales of hydrogen bonding: their construction and application to physicochemical and biochemical processes. Chem. Soc. Rev. 22, 73–83 (1993)
Abraham, M.H., Ibrahim, A., Zissimos, A.M.: The determination of sets of solute descriptors from chromatographic measurements. J. Chromatogr. A 1037, 29–47 (2004)
Abraham, M.H., Smith, R.E., Luchtefeld, R., Boorem, A.J., Luo, R., Acree, W.E. Jr.: Prediction of solubility of drugs and other compounds in organic solvents. J. Pharm. Sci. 99, 1500–1515 (2010)
Grunwald, E., Berkowitz, B.: The measurement and correlation of acid dissociation constants for carboxylic acids in the system ethanol–water. Activity coefficients and empirical activity functions. J. Am. Chem. Soc. 73, 4939–4944 (1951)
Spivey, H.O., Shedlovsky, T.: Studies of electrical conductance in alcohol–water mixtures. II. The ionisation constant of acetic acid in ethanol water mixtures at 0, 25, and 35 °C. J. Phys. Chem. 71, 2171–2175 (1967)
Frohlinger, J.O., Gartska, R.A., Irwin, H.W., Steward, O.W.: Determination of ionization constants of monobasic acids in ethanol–water solvents by direct potentiometry. Anal. Chem. 40, 1408–1411 (1968)
Dash, U.N., Pattanaik, E.R.: Conductometric determination of dissociation constants of carboxylic acids in various mixed solvents. Egypt. J. Chem. 37, 17–24 (1994)
Azab, H.A., Ahmed, L.T., Mahmoud, M.R.: Potentiometric determination of the dissociation constants of some monocarboxylic acids in various hydroorganic mixtures. J. Chem. Eng. Data 40, 523–525 (1995)
Thuaire, R.: Sur la dissociation des acides benzoiques substitues dans les melange eau–ethanol. J. Chim. Phys. 67, 1076–1087 (1970)
Niazi, M.S.K.: Conductivities and thermodynamic dissociation constants for chloroacetic acid in binary mixed solvent systems at 298.15 K. J. Chem. Eng. Data 38, 527–530 (1993)
Rubino, J.T., Berryhill, W.S.: Effects of solvent polarity on the acid dissociation constants of benzoic acids. J. Pharm. Sci. 75, 182–186 (1986)
Sarmini, K., Kenndler, E.: Capillary zone electrophoresis in mixed aqueous-organic media: effect of organic solvents on actual ionic mobilities, acidity constants and separation selectivity of substituted aromatic acids. II. Ethanol. J. Chromatogr. A 811, 201–209 (1998)
Menard, H., Lessard, J.: Acidities of some N-haloamides (ZCONHX) in water and ethanol–water mixtures at 25 °C. J. Chem. Eng. Data 23, 64–65 (1978)
Gutbbzahl, B., Grunwald, E.: The effect of solvent on equilibria and rate constants. II. The measurement and correlation of acid dissociation constants of anilinium and ammonium salts in the system ethanol–water. J. Am. Chem. Soc. 75, 559–565 (1953)
Reynaud, R.: Etudes experimentale de l’ionisation de quelqes amines aromatiques ou heterocycliques et des acides acetique et benzoique dans dix families de solvants mixtes. Bull. Soc. Chim. Fr. 1967, 4597–4604 (1967)
Oszczapowicz, J., Czuryłowska, M.: The pK a values of the conjugate acid of imidazole in water–ethanol mixtures. Talanta 31, 559–560 (1984)
Bell, R.P.: The Proton in Chemistry, Methuen & Co. Ltd., London (1959)
Seidell, A.: In: Linke, W.F. (ed.) Solubilities of Inorganic and Metal–Organic Compounds, vol. II, 4th edn. American Chemical Society, Washington (1965)
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Below are the links to the electronic supplementary material.
10953_2012_9822_MOESM1_ESM.doc
Equations for the Partition of Neutral Molecules, Ions and Ionic Species from Water to Water–Ethanol Mixtures (DOC 607 kB)
Rights and permissions
About this article
Cite this article
Abraham, M.H., Acree, W.E. Equations for the Partition of Neutral Molecules, Ions and Ionic Species from Water to Water–Ethanol Mixtures. J Solution Chem 41, 730–740 (2012). https://doi.org/10.1007/s10953-012-9822-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-012-9822-7