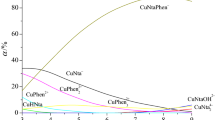
Abstract
The thermodynamics of dissociation of 3-(2-hydroxyphenylhydrazo)pentane-2,4-dione (H 2 L 1), 5,5-dimethyl-2-(2-hydroxyphenylhydrazo)cyclohexane-1,3-dione (H 2 L 2), 5,5-dimethyl-2-(2-hydroxy-4-nitrophenylhydrazo)cyclohexane-1,3-dione (H 2 L 3), 1-ethoxy-2-(2-hydroxyphenylhydrazo)butane-1,3-dione (H 2 L 4) and 1-ethoxy-2-(2-hydroxy-4-nitrophenylhydrazo)butane-1,3-dione (H 2 L 5) and of their complexation with copper(II) was studied in aqueous–ethanol solutions by potentiometry and UV–vis spectrophotometry. It was found that the thermodynamic parameters of the proton dissociation in H2L1–5 and of their complexation with copper(II) depend on the substituents in the aromatic and β-diketone fragments of the molecules. Thus, the acidic properties of H2L increase from H2L1 to H2L5, reflecting the electron-acceptor character of the substituents, whereas all of the thermodynamic functions tend to decrease with increasing electron-withdrawing capacity of the substituents. The complexation of Cu(II) with H2L1–5 is exothermic, which is connected with the formation of two stable chelating cycles.






Similar content being viewed by others
References
Aromi, G., Gamez, P., Reedijk, J.: Poly beta-diketones: prime ligands to generate supramolecular metalloclusters. Coord. Chem. Rev. 252, 964–989 (2008)
Vigato, P.A., Peruzzo, V., Tamburini, S.: The evolution of β-diketone or β-diketophenol ligands and related complexes. Coord. Chem. Rev. 253, 1099–1201 (2009)
Shchegol’kov, E.V., Burgart, Y.V., Khudina, O.G., Saloutin, V.I., Chupakhin, O.N.: 2-(Het)arylhydrazono-1,3-dicarbonyl compounds in organic synthesis. Russ. Chem. Rev. 79, 31–61 (2010)
Marten, J., Seichter, W., Weber, E.: A new carboxylic chelate ligand and its supramolecular complexes formed with sodium ions and alcohol molecules. Supramol. Chem. 22, 163–171 (2010)
Marten, J., Seichter, W., Weber, E.: 3-(Arylhydrazono)pentane-2,4-diones and their complexes with copper(II) and nickel(II)—synthesis and crystal structures. Z. Anorg. Allg. Chem. 631, 869–877 (2005)
Maharramov, A.M., Aliyeva, R.A., Mahmudov, K.T., Kurbanov, A.V., Askerov, R.K.: Copper(II) complex with 3-(2-hydroxy-3-sulfo-5-nitrophenylhydrazo)pentane-2,4-dione: synthesis and structure. Russ. J. Coord. Chem. 35, 704–709 (2009)
Mahmudov, K.T., Kopylovich, M.N., Guedes da Silva, M.F.C., Figiel, P.J., Karabach, Y.Y., Pombeiro, A.J.L.: New copper(II) dimer with 3-(2-hydroxy-4-nitrophenylhydrazo)pentane-2,4-dione and its catalytic activity in cyclohexane and benzyl alcohol oxidations. J. Mol. Catal. A, Chem. 318, 44–50 (2010)
Kopylovich, M.N., Mahmudov, K.T., Guedes da Silva, M.F.C., Kuznetsov, M.L., Figiel, P.J., Karabach, Y.Y., Luzyanin, K.V., Pombeiro, A.J.L.: Ortho-hydroxy substituted phenylhydrazo-β-diketones as valuable ligands. Catalytic ability of their copper(II) complexes towards oxidation of cyclohexane and benzyl alcohol. Inorg. Chem. 50, 918–931 (2011)
Kopylovich, M.N., Nunes, A.C.C., Mahmudov, K.T., Haukka, M., Mac Leod, T.C.O., Martins, L.M.D.R.S., Kuznetsov, M.L., Pombeiro, A.J.L.: Complexes of copper(II) with ortho-substituted 3-(phenylhydrazo)pentane-2,4-diones. Dalton Trans. 40, 2822–2836 (2011)
Li, Z., Mei, G., Zhang, H., Li, G., Wang, W., Zhang, Z.: Syntheses, characterizations and crystal structures of four zinc(II) and cadmium(II) complexes constructed by ligand bearing poly-coordination atoms. Inorg. Chim. Acta 363, 2616–2623 (2010)
Mei, G., Li, Z., Zhang, H., Li, G., Meng, X.: Synthesis, structures, and properties of two novel Zn(II) polynuclear complexes. Synth. Reac. Inorg. Met.-Org. Nano-Met. Chem. 40, 163–169 (2010)
Aliyeva, R.A., Chyragov, F.M., Mahmudov, K.T.: Dissociation constants for sulfoarylazo derivatives of acetylacetone and stability constants for their complexes. Russ. J. Inorg. Chem. 49, 1111–1113 (2004)
Inczedy, Y.: Analytical Application of Complex Equilibria. Akademiai Kiado, Budapest (1976)
Gadjieva, S.R., Mursalov, T.M., Mahmudov, K.T., Chyragov, F.M.: Quantum-chemical calculations of the tautomeric forms of 3-phenylazopentane-2,4-dione and the thermodynamic parameters of complexation between its isomers and some metals in aqueous ethanol. Russ. J. Coord. Chem. 32, 304–308 (2006)
Gadjieva, S.R., Mursalov, T.M., Mahmudov, K.T., Pashaev, F.G., Chyragov, F.M.: Complexation of copper(II) with 3-(2-hydroxyphenylazo)pentadione-2,4. J. Anal. Chem. 61, 550–555 (2006)
Bates, R.G.: Determination of pH, Theory and Practice. Wiley, New York (1973)
Bulatov, M., Kalinkin, I.: Manual on Photocalorimetric and Spectrophotometric Analytical Technique. Khimiya, Leningrad (1972)
Becke, A.D.: Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993)
Lee, C., Yang, W., Parr, R.G.: Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Zakrzewski, V.G., Montgomery, J.A. Jr., Stratmann, R.E., Burant, J.C., Dapprich, S., Millam, J.M., Daniels, A.D., Kudin, K.N., Strain, M.C., Farkas, O., Tomasi, J., Barone, V., Cossi, M., Cammi, R., Mennucci, B., Pomelli, C., Adamo, C., Clifford, S., Ochterski, J., Peterson, G.A., Ayala, P.Y., Cui, Q., Morokuma, K., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Cioslowski, J., Ortiz, J.V., Baboul, A.G., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Gomperts, R., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Andres, J.L., Gonzalez, C., Head-Gordon, M., Replogle, E.S., Pople, J.A.: Gaussian 98, revision A.9. Gaussian, Inc., Pittsburgh (1998)
Tomasi, J., Persico, M.: Molecular interaction in solution: an overview of methods based on continuous distribution of the solvent. Chem. Rev. 94, 2027–2094 (1994)
Barone, V., Cossi, M.: Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 102, 1995–2001 (1998)
Kopylovich, M.N., Mahmudov, K.T., Silva, M.F.C.G., Martins, L.M.D.R.S., Kuznetsov, M.L., Silva, T.F.S., Silva, J.J.R.F., Pombeiro, A.J.L.: Trends in properties of para-substituted 3-(phenylhydrazo)pentane-2,4-diones. J. Phys. Org. Chem. 24, 764–773 (2011)
Hansch, C., Leo, A., Taft, W.R.: A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 91, 165–195 (1991)
Dorokhova, E.N., Prokhorova, G.V.: Problems and Questions on Analytical Chemistry. Mir, Moscow (2001)
Astakhov, K.V., Verinikin, V.B., Zimin, V.I., Zvereva, A.D.: Spectrophotometric study of the complexation of some rare-earth metals with nitriloacetic acid. Russ. J. Inorg. Chem. 6, 2069–2071 (1961)
Podchainova, V.N., Simonova, L.N.: Analytical Chemistry of Copper. Nauka, Moscow (1990)
Courtney, R.C., Chaberek, S. Jr., Martell, A.E.: Stability of metal chelates. VII. N,N′-ethylenediaminedipropionic acid and N,N′-ethylenediaminetetrapropionic acid. J. Am. Chem. Soc. 75, 4814–4818 (1953)
Inczedy, J.: Analytical Application of Complex Equilibria. Akademiai Kiado, Budapest (1976)
Acknowledgements
This work has been partially supported by the Foundation for Science and Technology (FCT), Portugal, and its PPCDT (FEDER funded). K.T.M. expresses gratitude to the FCT for the post-doc fellowship. M.N.K. is grateful to the FCT and IST for a research contract within the Ciência 2008 scientific program.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below are the links to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mahmudov, K.T., Kopylovich, M.N., Kuznetsov, M.L. et al. Thermodynamics of Dissociation of ortho-Hydroxyphenylhydrazo-β-diketones and of Their Complexation with Copper(II) in Aqueous–Ethanol Solutions. J Solution Chem 41, 491–502 (2012). https://doi.org/10.1007/s10953-012-9816-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-012-9816-5