Abstract
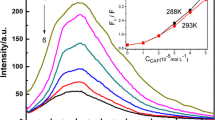
The interactions between pentachlorophenol (PCP) and jack bean urease were studied using UV/vis absorption, CD, fluorescence, synchronous fluorescence, and three-dimensional fluorescence spectroscopic techniques. The fluorescence data showed that the fluorescence quenching of urease by PCP the results of the formation of a PCP–urease complex involving a hydrophobic interaction. The distance r between the donor (urease) and acceptor (PCP) was obtained from the fluorescence resonance energy transfer. The effect of PCP on the conformation of urease was analyzed using UV/vis absorption, synchronous fluorescence and three-dimensional fluorescence spectroscopic techniques. The result showed that PCP can enter into the hydrophobic pocket at the interface of urease and that the micro environments around the tyrosine and tryptophan residues were changed.
Similar content being viewed by others
References
Krajewska, B., Ureases, I.: Functional, catalytic and kinetic properties: a review. J. Mol. Catal., B Enzym. 59, 9–21 (2009)
Krajewska, B., Zaborska, W., Chudy, M.: Multi-step analysis of Hg2+ ion inhibition of jack bean urease. J. Inorg. Biochem. 98, 1160–1168 (2004)
Jabri, E., Carr, M.B., Hausinger, R.P., Karplus, P.A.: The crystal structure of urease from Klebsiella aerogenes. Science 268, 998–1004 (1995)
Liu, H.J., Zhan, X.M., Li, K.B., Liu, W.P.: Study on the reaction mechanism of chloroacetanilide herbicides with urease using fluorescence spectrum and high performance liquid chromatography. Spectrosc. Spectr. Anal. 25, 463–466 (2005)
Zhang, L., Mulrooney, S.B., Leung, A.F.K., Zeng, Y., Ko, B.B.C., Hausinger, R.P., Sun, H.: Inhibition of urease by bismuth(III): Implications for the mechanism of action of bismuth drugs. BioMetals 19, 503–511 (2006)
Bourguignon, B., Marcenac, F., Keller, H.R., Aguiar, P.F., Massart, D.L.: Simultaneous optimization of pH and organic modifier content of the mobile phase for the separation of chlorophenols using a Doehlert design. J. Chromatogr. 628, 171–189 (1993)
Carrizo, D., Grimalta, J.O., Ribas-Fito, N., Torrent, M., Sunyer, J.: Pentachlorobenzene, hexachlorobenzene, and pentachlorophenol in children’s serum from industrial and rural populations after restricted use. Ecotoxicol. Environ. Saf. 71, 260–266 (2008)
Lenke, H., Pieper, D.H., Bruhn, C., Knackmuss, H.J.: Degradation of 2 4-dinitrophenol by two Rhodococcus erythropolis strains, HL 24-1 and HL 24-2. Appl. Environ. Microbiol. 58, 2928–2932 (1992)
Bukowska, M.: Effects of 2,4-D and its metabolite 2,4-dichlorophenol on antioxidant enzymes and level of glutathione in human erythrocytes. Comp. Biochem. Physiol. 135, 435–441 (2003)
Shen, D.S., Liu, X.W., Feng, H.J.: Effect of easily degradable substrate on anaerobic degradation of pentachlorophenol in an upflow anaerobic sludge blanket (UASB) reactor. J. Hazard. Mater. 119, 239–243 (2005)
Chang, B.V., Zheng, J.X., Yuan, S.Y.: Effect of alternative electron donors, acceptors and inhibitors on pentachlorophenol dechlorination in soil. Chemosphere 33, 313–320 (1996)
He, Y., Xu, J.M., Tang, C., Wu, Y.P.: Facilitation of pentachlorophenol degradation in the rhizosphere of ryegrass (Loliu perenne L). Soil. Biol. Biochem. 37, 2017–2024 (2005)
Lakowicz, J.R.: Principles of Fluorescence Spectroscopy. Plenum Press, New York (1999)
Pham, T.T.P., Oyama, T., Isse, T., Kawamoto, T.: Application of tryptophan fluorescence to assess sensitizing potentials of chemicals. Arch. Environ. Contam. Toxicol. 57, 427–436 (2009)
Sułkowska, A., Równicka, J., Poźycka, J., Bojko, B., Sułkowski, W.W.: The effect of concentration of guanidine hydrochloride on the sulfasalazine–serum albumin complex. J. Mol. Struct. 744, 775–779 (2005)
Lakowicz, J.R., Weber, G.: Quenching of fluorescence byoxygen: probe for structural fluctuations in macromolecules. Biochemistry 12, 4161–417 (1973)
Silva, D., Cortez-Moreira, M., Bastos, V.L.F.C., Bastos, J.C., Cortez, C.M.: The interaction of methyl-parathion with serum and albumin of the neo-tropical Piaractus mesopotamicu. Ecotoxicol. Environ. Saf. 73, 32–37 (2010)
Sharma, A., Schulman, S.G.: Introduction to Fluorescence Spectroscopy. Wiley, New York (1999)
Cao, H., Liu, Q.: Effects of temperature and common ions on binding of puerarin to BSA. J. Solution Chem. 38, 1071–1077 (2009)
Wang, Y.Q., Zhang, H.M., Zhang, G.C., Tao, W.H., Tang, S.H.: Binding of brucine to human serum albumin. J. Mol. Struct. 830, 840–845 (2007)
Du, W.H., Li, Z.F., Wang, B.H., Zhang, Y.M.: A study on the interaction between cisplatin and urease. Thermochim. Acta 333, 109–114 (1999)
Tang, B.P., Wang, Y.Q., Zhang, D.Z.: Studies on the interaction between benzidine and the hemocyanin from Chinese mitten crab Eriocheir japonica sinensis. Spectrochim. Acta, Part A, Mol. Biomol. Spectrosc. 73, 676–681 (2009)
Ross, P.D., Subramanian, S.: Thermodynamics of protein association reaction: forces contribution to stability. Biochemistry 20, 3096–3102 (1981)
Sklar, L.A., Hudson, B.S., Simoni, R.D.: Conjugate polyene fatty acids as fluorescent membrane probes. Biochemistry 16, 5100–5108 (1977)
Abou-Zied, O.K., Al-Shihi, O.I.K.: Characterization of subdomain IIA binding site of human serum albumin in its native, unfolded, and refolded states using small molecular probes. J. Am. Chem. Soc. 130, 10793–10801 (2008)
Vial, S., Ghanbajab, J., Forano, C.: Precipitation of Zn2Al LDH by urease enzyme. Chem. Commun. 3, 290–292 (2006)
Zhang, H.M., Wang, Y.Q., Jiang, M.L.: Fluorimetric study of interaction of C. I. Solvent Red 24 with haemoglobin. Dyes Pigm. 82, 156–163 (2009)
Abert, W.C., Gregory, W.M., Allan, G.S.: The binding interaction of coomassie blue with proteins. Anal. Biochem. 213, 407–413 (1993)
Zhang, Y.Z., Zhou, B., Zhang, X.P., Huang, P., Li, C.H., Liu, Y.: Interaction of malachite green with bovine serum albumin: determination of the binding mechanism and binding site by spectroscopic methods. J. Hazard. Mater. 163, 1345–1352 (2009)
Cao, S.H., Wang, D.D., Tian, X.Y., Chen, J.W.: Interaction between trans-resveratrol and serum albumin in aqueous solution. J. Solution Chem. 38, 1193–1202 (2009)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, YQ., Zhang, GC. & Zhang, HM. Study on the Interaction of Pentachlorophenol with Urease in Aqueous Solution by Multiple Spectroscopic Techniques. J Solution Chem 40, 458–469 (2011). https://doi.org/10.1007/s10953-011-9664-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-011-9664-8