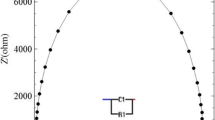
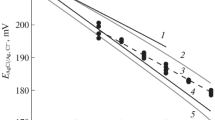
Abstract
Potentiometric cells from which the pH values of standard buffer solutions are computed rely on the silver–silver chloride reference electrode which has proved to be convenient, reproducible and reliable.
Of the various methods of preparation of silver-silver chloride electrodes only one concerns us here: the thermal–electrolytic type that has been used more extensively than any other form. Once prepared, the electrodes need to be equilibrated before use and between experiments. The equilibration technique must ensure voltage stability and inter-electrode, or bias, potential below 0.1 mV. In potentiometry the stability of a reference electrode is of utmost importance since an offset of 1 mV is equivalent to a deviation of about 0.02 in the pH value.
A thorough study has been conducted, involving a critical assessment of the various protocols which have been developed and adopted by different research schools and have been used in the course of inter-laboratory exercises. Factors that affect the electrode performance, such as size, mass, chloridization, storage and conditioning were systematically investigated, through measurements of bias potential, leading to a set of recommended procedures that meet the requirements for high quality results.
In this work, the adequacy of the optimized methodology is confirmed by means of primary pH measurements.
Similar content being viewed by others
References
Bates, R.G.: Determination of pH—Theory and Practice, 2nd edn. Wiley, New York (1973)
Buck, R.P., Rondinini, S., Covington, A.K., Baucke, F.G., Brett, C.M., Camões, M.F., Milton, M.J.F., Mussini, T., Naumann, R., Pratt, K.W., Spitzer, P., Wilson, G.S.: Measurement of pH. Definition, standards, and procedures—(IUPAC Recommendations 2002). Pure Appl. Chem. 74, 2169–2200 (2002)
IUPAC: Comparable pH measurements by metrological traceability. Current Project (Number: 2004-005-2-500), Analytical Chemistry Division (V)
Vyskočil, L., Máriássy, M., Mathiasová, A., Mendoza, M.M., Camoes, F., Vospělová, A., Kristensen, H.-B., Kozlowski, W., Kuselman, I., Hwang, E., Ivanova, D., Pratt, K.W., Spitzer, P.: Report of the Study CCQM-P37: Fundamental Studies of pH Standards. Slovak-Institute of Metrology, Bratislava (2002)
Damasceno, J.C., Borges, R.M.H., Ordine, A.P., Getrouw, M.A., Fraga, I.C.S., Borges, P.P., Souza, V.: Evaluation of Ag/AgCl-electrode standard potential uncertainty used in primary pH measurements by Monte Carlo simulation. Meas. Sci. Rev. 5, 49–52 (2005)
NMS: Standards and valid procedures to underpin measurements of pH and ionic content. Project P05—Proposal for the 2006–2009 VAM-Physical Programme
Ito, S., Hachiya, H., Baba, K., Asano, Y., Wada, H.: Improvement of the silver/silver chloride reference electrode and its application to pH measurement. Talanta 42, 1685–1690 (1995)
Durst, R.A.: Ion Selective Electrodes. National Bureaux of Standards Special Publication, vol. 314. NBS, Washington (1969)
Spitzer, P., Werner, B.: Improved reliability of pH measurements. Anal. Bioanal. Chem. 374, 787–795 (2002)
Meinrath, G., Ekberg, C., Landgren, A., Liljenzin, J.O.: Assessment of uncertainty in parameter evaluation and prediction. Talanta 51, 231–246 (2000)
Brown, R.J.C., Milton, M.J.T.: The microporous structure of silver/silver chloride electrodes and the implications for Harned cell operation. Accredit. Qual. Assur. 10, 352–355 (2005)
Brown, R.J.C., Milton, M.J.T.: Effect of structural design of silver/silver chloride electrodes on stability and response time and the implications for improved accuracy in pH measurement. Sensors 9, 118–130 (2009)
Guiomar, M.J., Lito, H.M., Camões, M.F., Viçoso, C.M.: Effect of condensation phenomena on potentiometric measurements. Anal. Chim. Acta 575, 45–51 (2006)
Guiomar, M.J., Lito, H.M., Camões, M.F., Viçoso, C.M.: Improving the quality of potentiometric pH measurements. Accredit. Qual. Assur. 12, 447–453 (2007)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lito, M.J.G., Camões, M.F. Meeting the Requirements of the Silver/Silver Chloride Reference Electrode. J Solution Chem 38, 1471–1482 (2009). https://doi.org/10.1007/s10953-009-9462-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-009-9462-8