Abstract
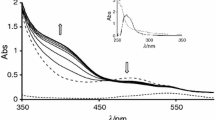
Ligand protonation and stepwise dissociation constants, formation constants and speciation of four pyridyl sulfonamide ligands (Congreeve et al., New J. Chem. 27:98–106, 2003) were assessed, using potentiometric and UV/Visible spectrophotometric pH titrations (in 80% MeOH − 20% H2O). The suitability of these ligands as Cu(II) and Zn(II) sensors for physiological applications was assessed. Two ligands L1 and L4 were p-toluenesulfonamide derivatives while L2 and L3 were triflurosulfonamide derivatives. Additionally L3 and L4 were appended with α-methyl groups. The most stable complex was formed by L1 with Cu(II) owing to the fact that this complex was square planar (log 10 K 1=12.15±0.004 and log 10 β 2=15.42±0.006). The rest of the complexes invariably formed distorted tetrahedron geometry and complexation was weaker. Speciation diagrams show the effect of ligand to metal concentration, revealing that the L2 and L3 ligands are the most suitable for forming ML2 complexes at physiological pH.
Similar content being viewed by others
References
Congreeve, A., Kataky, R., Knell, M., Parker, D., Puschmann, H., Senanayake, K., Wylie, L.: Examination of cobalt, nickel, copper and zinc (II) complex geometry and binding affinity in aqueous media using simple pyridylsulfonamide ligands. New J. Chem. 27, 98–106 (2003)
Rossi, L.M., Neves, A., Botoluzzi, A.J., Hörner, R., Szpoganicz, B., Terenzi, H., Mangrich, A.S., Pereira-Maia, E., Castellano, E.E., Haase, W.: Synthesis, structure and properties of unsymmetrical μ-alkoxo-dicopper(II) complexes: biological relevance to phosphodiester and DNA cleavage and cytotoxic activity. Inorg. Chim. Acta 358, 1807–1822 (2005)
Daniel, K.G., Gupta, P., Harbach, R.H., Guida, W.C., Dou, Q.P.: Organic copper complexes as a new class of proteasome inhibitors and apoptosis inducers in human cancer cells. Biochem. Pharmacol. 67, 1139–1151 (2004)
Choi, D.W., Koh, J.Y.: Zinc and brain injury. Ann. Rev. Neurosci. 21, 347–375 (1998)
Mitic, N., Smith, S.J., Neves, A., Guddat, L.W., Gahan, L.R., Schenk, G.: The catalytic mechanisms of binuclear metallohydrolases. Chem. Rev. 106, 3338–3363 (2006)
Kimber, M.C., Mahadevan, I.B., Lincoln, S.F., Ward, A.D., Tiekink, E.R.T.: The synthesis and fluorescent properties of analogues of the zinc(ii) specific fluorophore zinquin ester. J. Org. Chem. 65, 8204–8209 (2000)
Nasir, M.S., Fahrni, C.J., Suhy, D.A., Kolodsick, K.J., Singer, C.P., O’Halloran, T.V.: The chemical cell biology of zinc: structure and intracellular fluorescence of a zinc-quinolinesulfonamide complex. J. Biol. Inorg. Chem. 4, 775–783 (1999)
Hendrickson, K.M., Geue, J.P., Wyness, O., Lincoln, S.F., Ward, A.D.: Coordination and fluorescence of the intracellular Zn2+ probe [2-methyl-8-(4-toluenesulfonamido)-6-quinolyloxy]acetic acid (zinquin a) in ternary Zn2+ complexes. J. Am. Chem. Soc. 125, 3889–3895 (2003)
Fahrrni, C.J., O’Halloran, T.V.: Aqueous coordination chemistry of quinoline–based fluorescence probes for the biological chemistry of zinc. J. Am. Chem. Soc. 121, 11448–11458 (1999)
Nakamura, H., Yoshida, T., Todoka, M.: Syntheses and chelating properties of sulfonamidoquinolines. Bull. Chem. Soc. Jpn. 57, 2839–2846 (1984)
Congreeve, A.: Responsive lanthanide complexes for metal ion sensing. Doctoral Thesis, Department of Chemistry, University of Durham (2004)
Gans, P., Sabatini, A., Vacca, A.: SUPERQUAD: an improved general program for computation of formation constants from potentiometric data. J. Chem. Soc. Dalton Trans. 1195–1200 (1985)
Martell, A.E., Motekaitis, R.J.: Determination and Use of Stability Constants. VCH Publishers Inc., Cambridge (1992)
Rondinini, S., Mussini, P.R., Mussini, T.: pH measurements in non-aqueous and mixed solvents: Predicting pH(PS) of potassium hydrogen phthalate for alcohol-water mixtures (Technical Report). Pure Appl. Chem. 70, 1419–1422 (1998)
Marcus, Y., Mussini, T., Izutsu, K.: The extrapolation of EMF data to infinite dilution in non-aqueous and mixed solvents. Pure Appl. Chem. 63, 1647–1658 (1991)
Hakansson, K., Liljas, A.: The structure of a complex between carbonic anhydrase II and a new inhibitor, trifluoromethane sulphonamide. FEBS Lett. 350, 319–322 (1994)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kataky, R., Knell, M.A. Complexation Studies of Pyridyl Sulfonamide Ligands for Sensing Zinc and Copper Ions. J Solution Chem 38, 1483–1492 (2009). https://doi.org/10.1007/s10953-009-9456-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-009-9456-6