Abstract
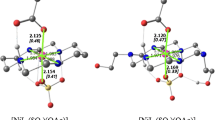
The formation constants of dioxouranium(VI)-2,2′-oxydiacetic acid (diglycolic acid, ODA) and 3,6,9-trioxaundecanedioic acid (diethylenetrioxydiacetic acid, TODA) complexes were determined in NaCl (0.1≤I≤1.0 mol⋅L−1) and KNO3 (I=0.1 mol⋅L−1) aqueous solutions at T=298.15 K by ISE-[H+] glass electrode potentiometry and visible spectrophotometry. Quite different speciation models were obtained for the systems investigated, namely: ML0, MLOH−, ML 2−2 , M2L2(OH)−, and M2L2(OH) 2−2 , for the dioxouranium(VI)–ODA system, and ML0, MLH+, and MLOH− for the dioxouranium(VI)–TODA system (M=UO 2+2 and L = ODA or TODA), respectively. The dependence on ionic strength of the protonation constants of ODA and TODA and of both metal-ligand complexes was investigated using the SIT (Specific Ion Interaction Theory) approach. Formation constants at infinite dilution are [for the generic equilibrium pUO 2+2 +q(L2−)+rH+ ⇌(UO 2+2 ) p (L) q H (2p−2q+r) r ;β pqr ]: log 10 β 110=6.146, log 10 β 11−1=0.196, log 10 β 120=8.360, log 10 β 22−1=8.966, log 10 β 22−2=3.529, for the dioxouranium(VI)–ODA system and log β 110=3.636, log 10 β 111=6.650, log 10 β 11−1=−1.242 for dioxouranium(VI)–TODA system. The influence of etheric oxygen(s) on the interaction towards the metal ion was discussed, and this effect was quantified by means of a sigmoid Boltzman type equation that allows definition of a quantitative parameter (pL 50) that expresses the sequestering capacity of ODA and TODA towards UO 2+2 ; a comparison with other dicarboxylates was made. A visible absorption spectrum for each complex reaching a significant percentage of formation in solution (KNO3 medium) has been calculated to better characterize the compounds found by pH-metric refinement.
Similar content being viewed by others
References
Miyazaki, M., Shimoishi, Y., Miyata, H., Tôei, K.: The reaction of dicarboxylic acids containing ether linkages with alkaline earth metals. J. Inorg. Nucl. Chem. 36, 2033–2038 (1974)
Maupin, C.L., Smith, K.C., Riehl, J.P.: Quantitative determination of Eu(III) complex speciation in aqueous complexes of Eu(III) with oxydiacetic acid using 5D0→7F0 excitation spectroscopy. J. Alloys Compd. 249, 181–184 (1997)
De Stefano, C., Gianguzza, A., Piazzese, D., Sammartano, S.: Speciation of low molecular weight carboxylic ligands in natural fluids: protonation constants and association with major components of seawater of oxydiacetic and citric acids. Anal. Chim. Acta 398, 103–110 (1999)
Jiang, J., Renshaw, J.C., Sarsfield, M.J., Livens, F.R., Collison, D., Charnock, J.M., Eccles, H.: Solution chemistry of uranyl ion with iminodiacetate and oxydiacetate: a combined NMR/EXAFS and potentiometry/calorimetry study. Inorg. Chem. 42, 1233–1240 (2003)
Rao, L., Garnov, A.Y., Jiang, J., Di Bernardo, P., Zanonato, P.L., Bismondo, A.: Complexation of uranium(VI) and samarium(III) with oxydiacetic acid: temperature effect and coordination modes. Inorg. Chem. 42, 3685–3692 (2003)
Berto, S., Crea, F., Daniele, P.G., De Stefano, C., Prenesti, E., Sammartano, S.: Dioxouranium(VI)–carboxylate complexes. Interaction with dicarboxylic acids in aqueous solution: speciation and structure. Ann. Chim. 96, 399–420 (2006)
Crea, F., De Robertis, A., Sammartano, S.: Dioxouranium carboxylates complexes. Formation and stability of acetate species at different ionic strengths in NaClaq. Ann. Chim. (Rome) 93, 1027–1035 (2003)
Crea, F., De Robertis, A., De Stefano, C., Sammartano, S.: Dioxouranium(VI)-carboxylate complexes. A calorimetric and potentiometric investigation on the interaction towards oxalate at infinite dilution and in NaCl aqueous solution at I=1.0 mol⋅L−1 and t=25 °C. Talanta 71, 948–963 (2007)
Crea, F., De Robertis, A., De Stefano, C., Sammartano, S.: Dioxouranium(VI)-carboxylate complexes. Interaction of UO 2+2 with 1,2,3,4,5,6-benzenehexacarboxylate (mellitate) in 0 ≤ I(NaClaq)≤ 1.0 mol⋅L−1. J. Solution Chem. 36, 479–496 (2007)
Crea, F., De Stefano, C., Milea, D., Sammartano, S.: Dioxouranium(VI)–carboxylate complexes. Speciation of UO 2+2 –1,2,3-propanetricarboxylate system in NaClaq at different ionic strengths and at t=25 °C. Ann. Chim. 97, 163–175 (2007)
Crea, F., Foti, C., Sammartano, S.: Sequestering ability of polycarboxylic ligands towards dioxouranium(VI). Talanta 75, 775–785 (2008)
Perrin, D.D., Armorego, W.L., Perrin, D.R.: Purification of Laboratory Chemicals. Pergamon, Oxford (1966)
De Stefano, C., Trinci, P., Rigano, C., Sammartano, S.: Computer analysis of equilibrium data in solution. ESAB2M: an improved version of the ESAB program. Ann. Chim. (Rome) 77, 643–675 (1987)
De Stefano, C., Mineo, P., Rigano, C., Sammartano, S.: Ionic strength dependence of formation constants. XVII. The calculation of equilibrium concentrations and formation constants. Ann. Chim. (Rome) 83, 243–277 (1993)
Gans, P., Sabatini, A., Vacca, A.: Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 43, 1739–1753 (1996)
Biedermann, G.: Ionic media, in Dahlem workshop on the nature of seawater. In: Dahlem Konferenzen, Berlin, pp. 339–362 (1975)
Ciavatta, L.: The specific interaction theory in evaluating ionic equilibria. Ann. Chim. (Rome) 70, 551–567 (1980)
De Stefano, C., Mineo, P., Rigano, C., Sammartano, S.: Computer tools for the speciation of natural fluids. In: Gianguzza, A., Pelizzetti, E., Sammartano, S. (eds.) Marine Chemistry—An Environmental Analytical Chemistry Approach, p. 71. Kluwer Academic, Amsterdam (1997)
Setschenow, J.Z.: Uber die konstitution der salzlosungenauf grund ihres verhaltens zu kohlensaure. Z. Phys. Chem. 4, 117–125 (1889)
Bretti, C., Foti, C., Porcino, N., Sammartano, S.: SIT parameters for 1:1 electrolytes and correlation with Pitzer coefficients. J. Solution Chem. 35, 1401–1415 (2006)
Crea, F., De Stefano, C., Gianguzza, A., Piazzese, D., Sammartano, S.: Protonation of carbonate in aqueous tetraalkylammonium salts at 25 °C. Talanta 68, 1102–1112 (2006)
De Stefano, C., Gianguzza, A., Leggio, T., Sammartano, S.: Dependence on ionic strength of hydrolysis constants for dioxouranium(VI) in NaCl(aq) and NaNO3(aq), at pH<6 and t=25 °C. J. Chem. Eng. Data 47, 533–538 (2002)
Gianguzza, A., Milea, D., Millero, F.J., Sammartano, S.: Hydrolysis and chemical speciation of dioxouranium(VI) in aqueous media simulating the major ion composition of seawater. Mar. Chem. 85, 103–124 (2004)
De Stefano, C., Gianguzza, A., Piazzese, D., Sammartano, S.: Speciation of low molecular weight carboxylic ligands in natural fluids: protonation constants and association with major components of seawater of oxydiacetic and citric acids. Anal. Chim. Acta 398, 103–110 (1999)
De Stefano, C., Gianguzza, A., Piazzese, D.: Complexes of azelaic and diethylenetrioxydiacetic acids with Na+, Mg2+ and Ca2+ in NaCl aqueous solutions, at 25 °C. J. Chem. Eng. Data 45, 15–19 (2000)
Martell, A.E., Motekaitis, R.J., Smith, R.M.: NIST-Database 46. Gaithersburg (1997)
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Berto, S., Crea, F., Daniele, P.G. et al. Sequestering Ability of Dicarboxylic Ligands Towards Dioxouranium(VI) in NaCl and KNO3 Aqueous Solutions at T=298.15 K. J Solution Chem 38, 1343–1356 (2009). https://doi.org/10.1007/s10953-009-9452-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-009-9452-x