Abstract
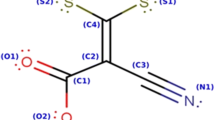
Electromotive force (E) measurements have been made at 303.15 K on the cell, Cu x Hg/CuCl2 (m) in solvent S′ [S′=W or FD or DMF each containing 15 ppm of 1,2,3-benzotriazole (BTA)] /AgCl/Ag (where W, FD and DMF represent the solvents water, formamide and N,N-dimethylformamide, respectively). The Emf data have been compared with the corresponding data under the same conditions in pure W, FD and DMF; the Emf values change from negative to very positive in the presence of BTA in these solutions and suggest the possibility that the presence of BTA in W, FD and DMF helps to bind copper ions with solvent molecules through formation of new complex structures of the film formed on the metal surface. We have suggested the probable mechanism of formation of a protective film through complex formation. The higher Emf values in the presence of BTA indicate strong ion-solvent interactions and the order of interactions was found to be as: FD≥DMF>W. Further iterative procedures were employed to evaluate K 1,K 2 (dissociation constants), α 1,α 2 (degrees of dissociation), γ ± (mean activity coefficient) and E o (standard cell potential) making use of the measured E values. The standard molar Gibbs energy of transfer, ΔG otr , of CuCl2 from the pure S to S′ were calculated using the computed standard cell potentials. The calculated ΔG otr values were found to be negative which was indicative of strong ion-solvent interactions and reasonably supported the trend of ion-solvent interactions. From these studies we concluded that BTA acts as a better corrosion inhibitor towards copper in FD and DMF than water.
Similar content being viewed by others
References
Oguzie, E.E., Li, Y., Wang, F.H.: Effect of 2-amino-3-mercaptopropanoic acid (cysteine) on the corrosion behaviour of low carbon steel in sulphuric acid. Electrochim. Acta 53, 909–914 (2007)
Mu, G., Li, X.: Inhibition of cold rolled steel corrosion by Tween-20 in sulphuric acid: Weight loss, electrochemical and AFM approach. J. Colloid Interface Sci. 289, 184–192 (2005)
Larabi, L., Harek, Y., Benali, O., Ghalem, S.: Hydrazine derivatives as corrosion inhibitors for mild steel in 1 M HCl. Prog. Org. Coat. 54, 256–262 (2005)
Oguzie, E.E., Unaegbu, C., Ogukwe, C.N., Onuchukwu, A.I.: Inhibition of mild steel corrosion in sulphuric acid using indigo dye and synergistic halide additives. Mater. Chem. Phys. 84, 363–368 (2004)
Oguzie, E.E., Li, Y., Onuoha, G.N., Onuchukwu, A.I.: Inhibitory mechanism of mild steel corrosion in 2 M sulphuric acid solution by methylene blue dye. Mater. Chem. Phys. 89, 305–311 (2004)
Feng, Y., Siow, K.S., Teo, W.K., Hsieh, A.K.: The synergic effects of propargyl alcohol and potassium iodide on the inhibition of mild steel in 0.5 M sulphuric acid solution. Corros. Sci. 41, 829–852 (1999)
Riggs, O.L. Jr.: Corrosion Inhibitors, 2nd edn. Nathan, C.C., Houston (1973)
Sherif, E.M., Erasmus, R.M., Comins, J.D.: Effects of 3-amino-1,2,4-triazole on inhibition of copper corrosion in acidic chloride solutions. J. Colloid Interface Sci. 311, 144–151 (2007)
Popva, A., Christov, M.: Evaluation of impedance measurements on mild steel corrosion in acid media in the presence of heterocyclic compounds. Corros. Sci. 48, 3208–3221 (2006)
Khaled, K.F.: The inhibition of benzotriazole derivative on corrosion of iron in 1 M HCl solution. Electrochim. Acta 48, 2493–2503 (2003)
Frignani, A., Trabanelli, G.: Influence of organic additives on the corrosion of ion-based amorphous alloys in dilute sulphuric acid solution. Corrosion 55, 653–660 (1999)
Wang, H.L., Liu, R.B., Xin, J.: Inhibitory effects of some mercapto-triazole derivatives on the corrosion of mild steel in 1 M HCl medium. Corros. Sci. 46, 2455–2466 (2004)
Qafsaoui, W., Blanc, C., Pebere, N., Takenouti, H., Srhiri, A., Mankowski, G.: Quantitative characterization of protective films grown on copper in the presence of different triazole derivative inhibitors. Electrochim. Acta 47, 4339–4346 (2002)
Tromans, D.: Aqueous potential-pH equilibria in copper-benzotriazole systems. J. Electrochem. Soc. 145, L42–L45 (1998)
Sutter, E.M.M., Fiaud, C., Lincot, D.: Electrochemical and photoelectrochemical characterization of naturally grown oxide layers on copper in sodium acetate solutions with and without benzotriazole. Electrochim. Acta 38, 1471–1479 (1993)
Tommesani, L., Brunoro, G., Frignani, A., Monticelli, C., Dal Colle, M.: On the protective action of 1,2,3-benzotriazole derivative films against copper corrosion. Corros. Sci. 39, 1221–1237 (1997)
Ferina, S., Loncar, M., Metikos-Hukovik, M.: In: Proceedings of the 8th Symposium on Corrosion Inhibitors, Ferrara, Italy, p. 1065 (1995)
Babic, R., Metikos-Hukovik, M., Loncar, M.: Impedance and photoelectrochemical study of surface layers on Cu and Cu-10Ni in acetate solution containing benzotriazole. Electrochim. Acta 44, 2413–2421 (1999)
Sayed, S.Y., El-Deab, M.S., El-Anadouli, B.E., Ateya, B.G.: Synergetic effects of benzotriazole and copper ions on the electrochemical impedance spectroscopy and corrosion behaviour of iron in sulphuric acid. J. Phys. Chem. B 107, 5575–5585 (2003)
Walsh, J.F., Dhariwal, H.S., Gutierrez-Sosa, A., Finetti, P., Muryn, C.A., Brookes, N.B., Oldman, R.J., Thornton, G.: Probing molecular orientation in corrosion inhibition via a NEXAFS study of benzotriazole and related molecules on Cu(100). Surf. Sci. 415, 423–432 (1998)
Lewis, G.: The corrosion inhibition of copper by benzimidazole. Corros. Sci. 22, 579–584 (1981)
Fang, B.S., Olson, C.G., Lynch, D.W.: A photoemission study of benzotriazole on clean copper and cuprous oxide. Surf. Sci. 176, 476–490 (1986)
Vogt, M.R., Nichols, R.J., Magnussen, O.M., Behm, R.J.: Benzotriazole adsorption and inhibition of Cu(100) corrosion in HCl: A combined in situ STM and in situ FTIR spectroscopy study. J. Phys. Chem. B 102, 5859–5865 (1998)
Rubim, J., Gutz, I.G.R., Sala, C., Orville-Thomas, J.: Surface enhanced Raman spectra of benzotriazole adsorbed on a copper electrode. J. Mol. Struct. 100, 571–583 (1983)
Poling, G.W.: Reflection infra-red studies of films formed by benzotriazole on Cu. Corros. Sci. 10, 359–370 (1970)
EL-Taib Heakal, F., Haruyama, S.: Impedance studies of the inhibitive effect of benzotriazole on the corrosion of copper in sodium chloride medium. Corros. Sci. 20, 887–898 (1980)
Aramaki, K., Kiuchi, T., Sumiyoshi, T., Nishihara, H.: Surface enhanced Raman scattering and impedance studies on the inhibition of copper corrosion in sulphate solutions by 5-substituted benzotriazoles. Corros. Sci. 32, 593–607 (1991)
Glasstone, S.: An Introduction to Electrochemistry, 4th edn, p. 250. Van Nostrand East-West, New Delhi (1974)
Singh, P.P., Soni, M., Maken, S.: Solvation behaviour of cupric chloride in non-aqueous solvents. Indian J. Chem. 31A, 227–231 (1992)
Mayanna, S.M., Setty, T.V.H.: Effect of halide ions on the dissolution of copper single crystal planes in dilute sulphuric acid. Corros. Sci. 14, 691–699 (1975)
Ross, J.K., Berry, M.R.: Benzotriazole as an inhibitor of the corrosion of Cu in flowing H2SO4. Corros. Sci. 11, 273–274 (1971)
Mansfield, F., Smith, T.: Benzotriazole as corrosion inhibitor for copper: part 2-acid NaCl solutions. Corrosion 29, 105–107 (1973)
Singh, P.P., Bhatia, M.: Topological investigations of the state of a salt in some binary mixtures of non-electrolytes. J. Chem. Soc. Faraday Trans. I 85, 3797–3805 (1989)
Andrew, A.W., Armitage, D.A., Broadband, R.W.C., Morcom, K.W., Muju, B.L.: Solubility of cadmium chloride and the standard potential in formamide. Trans. Faraday Soc. 67, 128–131 (1971)
Hefley, J.D., Amis, E.S.: Electromotive force studies of cadmium chloride in water, water-ethanol, and ethanol solutions. J. Phys. Chem. 69, 2082–2089 (1965)
Harned, H.S., Owen, B.B.: Physical Chemistry of Electrolytic Solutions. Reinhold, New York (1967)
Singh, P.P., Soni, M.: Solvation behaviour of copper(II) chloride in some isodielectric media. Indian J. Chem. 32A, 837–841 (1993)
Bose, K., Dass, A.K., Kundu, K.K.: Free energies and entropies of transfer of hydrobromic and hydroiodide acids from water to t-butyl alcohol + water mixtures from electromotive force measurements at different temperatures (5–35°C). J. Chem. Soc. Faraday Trans. 1 71, 1838–1848 (1975)
Chaudhary, M., Persson, I.: Transfer thermodynamic study on the copper (II) ion from water to methanol, acetonitrile, dimethyl sulfoxide and pyridine. J. Chem. Soc. Faraday Trans. 90, 2243–2248 (1994)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spah, M., Spah, D.C., Singh, K.C. et al. Effect of Solvents on the Corrosion Inhibition of Copper by 1,2,3-Benzotriazole. J Solution Chem 37, 1197–1206 (2008). https://doi.org/10.1007/s10953-008-9303-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-008-9303-1