Abstract
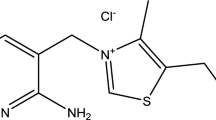
Effects of sulfathiazole (ST) on copper corrosion as a corrosion inhibitor in 0.1 M NaCl solutions have been studied using potentiodynamic polarization, open circuit potential, electrochemical impedance spectroscopy with scanning electron microscopy (SEM). Potentiodynamic polarization measurements indicated that the presence of ST in chloride solutions affects mainly the cathodic process and decreases the corrosion current and shifts the corrosion potential towards more negative values. The adsorption of inhibitor on the copper surface obeys the Langmuir adsorption isotherm. The adsorption free energy of ST on copper (−33.47 kJ/mol) shows a strong adsorption of the inhibitor on the metal surface. The effect of temperature on the inhibition efficiency of sulfathiazole was examined with Arrhenius equation and activation energies in 0.1 M NaCl with and without inhibitor were calculated. Impedance data were analyzed using an appropriate equivalent circuit model for the electrode /electrolyte interface. SEM measurements also exhibited that the ST molecules are strongly adsorbed on the copper surface.
Similar content being viewed by others
References
Nu’n’ez, L., Reguera, E., Corvo, F., et al., Corr. Sci., 2005, vol. 147, p. 461.
Lalitha, A., Ramesh, S., and Rajeswari, S., Electrochim. Acta, 2005, vol. 51, p. 47.
Zhang, D., Gao, L., and Zhou, G., Appl. Surf. Sci., 2004, vol. 225, p. 287.
Khaled, K.F. and Hackerman, N., Electrochim. Acta, 2004, vol. 49, p. 485.
Khaled, K.F., Appl. Surf. Sci., 2008, vol. 255, p. 1811.
Antonijevič, M.M., Milič, S.M., and Petrovič, M.B., Corr. Sci., 2009, vol. 51, p. 1228.
Larabi, L., Benali, O., Mekelleche, S., and Harek, Y., Appl. Surf. Sci., 2006, vol. 253, p. 1371.
Zor, S., Saracoglu, M., Kandemirli, F., and Arslan, T., Corrosion, 2011, vol. 67, p. 125003-1.
El Warraky, A., El Shayeb, H.A., and Sherif, E.M., Anti-Corrosion Methods and Materials, 2004, vol. 51, p. 52.
Lee, H.P. and Nobe, K., J. Electrochem. Soc., 1986, vol. 133, p. 2035.
Yu, P., Liao, D.-M., Luo, Y.-B., and Chen, Z.-G., Corrosion, 2003, vol. 59, p. 314.
Da Costa, S.L.F.A., Agostinho, S.M.L., and Nobe, K., J. Electrochem. Soc., 1993, vol. 140, p. 3483.
Lewis, G., Corr. Sci., 1982, vol. 22, p. 579.
Zucchi, F., Trabanelli, G., and Fonsati, M., Corr. Sci., 1996, vol. 38, p. 2019.
Raja, P.B. and Sethuraman, M.G., Mater. Lett., 2008, vol. 62, p. 113.
Newman, D.J. and Cragg, G.M., J. Nat Prod., 2007, vol. 70, p. 461.
Fouda, A.S., Mostafa, H.A., and El-Abbasy, H.M., J. Appl. Electrochem., 2010, vol. 40, p. 163.
Samide, A., Tutunaru, B., Negrila, C., et al., J. Nanomater. Bios., 2011, vol. 6, p. 663.
Samide, A., Tutunaru, B., Negrila, C., and Prunaru, I., Spectroscopy Lett., 2012, vol. 45, p. 55.
El-Naggar, M.M., Corr. Sci., 2007, vol. 49, p. 2226.
Abdallah, M., Corr. Sci., 2004, vol. 46, p. 1981.
Ebenso, E.E., Arslan, T., Kandemirli, F., Love, I., et al., Int. J. Quant. Chem., 2010, vol. 110, p. 2614.
Gece, G., Corr. Sci., 2011, vol. 53, p. 3873.
Ismail, K.M., Electrochim. Acta, 2007, vol. 52, p. 7811.
Diard, J.P., Le Canut, J.M., Le Gorrec, B., and Montella, C., Electrochim. Acta, 1998, vol. 43, p. 2469.
Crundwell, F.K., Electrochim. Acta, 1991, vol. 36, p. 2135.
Khaled, K.F., Mater. Chem. Phys., 2008, vol. 112, p. 104.
Allabergenov, K.D. and Kurbanov, F.K., Zashch Met., 1979, vol. 15, p. 472.
Sherif, E.M. and Park, S.M., Corr. Sci., 2006, vol. 48, p. 4065.
Schmitt, G., Brit. Corr. J., 1984, vol. 19, p. 165.
Liao, Q.Q., Yue, Z.W., Yang, D., et al., Corr. Sci., 2011, vol. 53, p. 1999.
Hu, L., Zhang, S., Li, W., and Hou, B., Corr. Sci., 2010, vol. 52, p. 2891.
Obot, I.B., Obi-Egbedi, N.O., and Umoren, S.A., Corr. Sci., 2009, vol. 51, p. 1868.
Obot, I.B. and Obi-Egbedi, N.O., Corr. Sci., 2010, vol. 52, p. 198.
Popova, A., Sokolova, E., Raicheva, S., and Christov, M., Corr. Sci., 2003, vol. 45, p. 33.
Li, S.L., Wang, Y.G., Chen, S.H., and Yu, R., Corr. Sci., 1999, vol. 41, p. 1769.
Sherif, E.M. and Park, S.M., Electrochim. Acta, 2006, vol. 51, p. 4665.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Zor, S. Sulfathiazole as potential corrosion inhibitor for copper in 0.1 M NaCl. Prot Met Phys Chem Surf 50, 530–537 (2014). https://doi.org/10.1134/S2070205114040200
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205114040200