Abstract
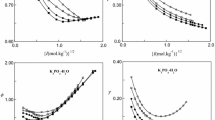
Isopiestic measurements have been carried out at the temperature 298.15 K for the quinary system (water + mannitol(sat) + sodium chloride + ammonium chloride + barium chloride) saturated with mannitol and its ternary sub-systems (water + mannitol(sat) + sodium chloride), (water + mannitol(sat) + ammonium chloride) and (water + mannitol(sat) + barium chloride). Taking aqueous sodium chloride as reference solutions, osmotic coefficients of the other aqueous solutions were determined. The experimental results show that the isopiestic activities of the quinary system in relation to its ternary sub-systems are in excellent agreement with the ideal-like solution model.
Similar content being viewed by others
References
Z. C. Wang, Ber. Bunsenges. Phys. Chem. 102, 1045 (1998).
X. W. Jin and Z. C. Wang, J. Chem. Thermodyn. 32, 349 (2000).
M. Wang, D. M. Yang, S. S. Sun, and Z. C. Wang, J. Chem. Thermodyn. 32, 389 (2000).
M. Wang, H. Zhang, D. M. Yang, and Z. C. Wang, J. Chem. Thermodyn. 33, 711 (2001).
M. Wang, Z. C. Wang, D. M. Yang, and J. Wang, J. Chem. Thermodyn. 34, 1575 (2002).
J. A. Rard and R. F. Platford, Activity Coefficients in Electrolyte Solutions, 2nd edn. (CRC Press, Boca Raton, FL, 1991), Chapter 5.
J. A. Rard, S. L. Clegg, and D. A. Palmer, J. Solution Chem. 29, 1 (2000).
Z. C. Wang, H. L. Yu, and Y. F. Hu, J. Chem. Thermodyn. 26, 171 (1994).
Y. F. Hu and Z. C. Wang, J. Chem. Thermodyn. 26, 429 (1994).
Y. F. Hu and Z. C. Wang, J. Chem. Thermodyn. 29, 879 (1997).
Z. C. Wang, X. Y. Li, and Y. H. Liu, J. Chem. Thermodyn. 30, 709 (1998).
Z. C. Wang, X. H. Zhang, T. Z. He, and Y. H. Bao, J. Chem. Soc., Faraday Trans. 84, 4369 (1988).
Z. C. Wang, X. H. Zhang, T. Z. He, and Y. H. Bao, J. Chem. Thermodyn. 21, 653 (1989).
X. H. Zhang, T. Z. He, Y. H. Bao, and Z. C. Wang, Acta Metall. Sinica B 3, 63 (1990).
Z. C. Wang, X. H. Zhang, and J. K. Zhou, Metal. Trans. B 23, 623 (1992).
Z. C. Wang, Y. W. Tian, H. L. Yu, and J. K. Zhou, Metal. Trans. B 23, 666 (1992).
Y. W. Tian, H. L. Yu, Z. C. Wang, X. H. Zhang, and J. K. Zhou, J. Chem. Thermodyn. 25, 711 (1993).
Z. C. Wang, Y. W. Tian, H. L. Yu, X. H. Zhang, and J. K. Zhou, Metal. Mater. Trans. B 25, 103 (1994).
D. G. Archer, J. Phys. Chem. Ref. Data 21, 793 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wang, J., Wang, ZC., Li, JL. et al. Isopiestic Study of Water + Mannitol(sat) + Sodium Chloride + Ammonium Chloride + Barium Chloride at T = 298.15 K and Comparison with the Ideal-Like Solution Model. J Solution Chem 34, 369–373 (2005). https://doi.org/10.1007/s10953-005-3057-9
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s10953-005-3057-9