Abstract
In this study, we presented two reliable methods, the sol–gel and autocombustion, to synthesis a high purity BiFeO3 single phase with low calcination time using glycine as chelating agent. The glycine-autocombustion method produced a high purity BiFeO3 phase using either low or high concentrations of the reactants (Bi(NO3)3 + Fe(NO3)3), while the glycine-sol–gel method delivered a high purity BiFeO3 phase using low concentrations of reactants. In the case of using tartaric acid and urea as chelating agents, mixtures of BiFeO3 and Bi25FeO40 phases were formed. The morphology, size, and porosity of the particles were obviously changed by varying the synthesis method and chelating agents. The high purity BiFeO3 samples exhibit a visible light band gap of 2.05 eV with long absorption tail extending to the infrared region, suggesting the suitability of the synthesized powders in the solar photocatalytic applications. A weak hysteresis ferromagnetic loop was observed for BiFeO3 (glycine method) and BiFeO3/Bi25FeO40 (urea method) with large contribution from the paramagnetic behavior. On contrast, robust ferromagnetic loops were found for BiFeO3/Bi25FeO40 sample synthesized by tartaric acid with saturation magnetization reaching to 2.5 emu/g. Remarkably, the pure single phase BiFeO3 powders synthesized by sol–gel and auto-combustion methods using glycine possess room temperature dielectric constant values of 622 and 845 respectively at a frequency of 42 Hz. In the case of BiFeO3 powders prepared by using tartaric acid, the dielectric constant exhibits values of 401 and 1118 for sol–gel and auto-combustion assisted samples, at the same frequency, respectively. At low frequency, the values of the real part of the complex permittivity tend to be zero which confirms a negligibly small contribution of the electrode effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Developing the magnetoelectric properties of multiferroic BiFeO3 is one of the reliable ways to commercialize spin-based devices like memory chips [1,2,3]. Magnetic and ferroelectric random access memories (M-RAM, Fe-RAM) are new separated classes of commercial memory devices which operate based on ferromagnetic and ferroelectric properties, respectively [4,5,6]. Although each of the magnetic and the ferroelectric random access memories has separated advanced properties, they, unfortunately, have some defects [4,5,6]. Combined of M-RAM and Fe-RAM in a single memory is a world challenge issue, leading to innovative and ultimate memory devices [4,5,6]. One of the basic requirements for attaining this step is to produce materials that can pair the advanced room temperature ferromagnetic-ferroelectric properties in a single structure [4,5,6]. For this purpose, BiFeO3 is a strong candidate for combining these properties at room temperature [4,5,6]. Multiferroic BiFeO3 has ferroelectric properties under Curie temperature (TC) of ~ 830 °C and G-type antiferromagnetic order below Néel temperature (TN) of ~ 370 °C [7,8,9]. In the recent decade, BiFeO3 is considered as one of the top-investigated single-phase multiferroic materials [4]. These condensed investigations aimed to overcome the synthesis obstacles and enhance the BiFeO3 properties to meet the spin-based application requirements [4]. One of the basic obstacles related to BiFeO3 is to obtain a pure single-phase structure without the generation of impurities such as Bi2Fe4O9, Bi2O3, and Bi25FeO39 [10,11,12]. Synthesizing of bulk or nanoparticle powders or thin-film of pure single phase BiFeO3 is a challenging task [13]. Scientists investigated various physical and chemical pathways to achieve this purpose [14]. Numerous research attempts were employed to develop efficient, simple, and inexpensive chemical methods to synthesize a high purity BiFeO3 [15]. Chien et al. reported the influences of numerous synthesis routes on photoelectrochemical and photocatalytic characteristics of single-phase perovskite BiFeO3 [16]. The authors found the formation of single phase BiFeO3 structure with remarkable magnetic and sunlight photocatalytic properties. Other studies on the synthesis and characterization of pure single phase BiFeO3 for biological and wastewater treatment were carried out by different authors [17,18,19]. Sol–gel and autocombustion are two easy synthesize methods with low requirements, and preparation of BiFeO3 by both techniques is favorable due to low-cost production. The sol–gel and auto-combustion techniques offer several advantages including the homogenous mixing of metal cations of the required composition at a molecular scale, high crystallinity and purity of the products, and small and narrow particle size distribution. Also, stoichiometry controlling and the introduction of dopants into the final product are convenient. Furthermore, the two techniques involve simple preparation processes, low consumption of external energy, and no complicated handlings are involved. Ortiz-Quiñonez et al. [20] reported a successful synthesis of pure BiFeO3 nanoparticles using a glycine-assisted route. However, the authors stated that the formation of the pure phase of BiFeO3 needed calcination time for the as-synthesized powder for a long period (1 h at 350 °C, 6 h at 500 °C, and 1 h at 600 °C) [20]. In the present study, the authors successfully and powerfully reduced the calcination time for synthesized single BiFeO3 powder (glycine route) to only 2 h to attain a high purity BiFeO3 (1 h at 500 °C and 1 h at 600 °C). For saving time and energy, the total calcination time was reduced from 8 [20] to 2 h only in the present work. Besides, the authors prepared a single phase BiFeO3 using low and high reactant concentrations for large amount production. Herein, we study the synthesis of pure BiFeO3 phase by two methods including sol–gel and autocombustion using three chelating agents (glycine, tartaric acid, and urea). The structure, morphology, optical, magnetic, and electrical properties of the synthesized samples were investigated and discussed. The obtained results show that glycine-autocombustion method produced a high purity BiFeO3 phase using either low or high concentrations of the reactants (Bi(NO3)3 + Fe(NO3)3), while the glycine-sol–gel method delivered a high purity BiFeO3 phase using low concentrations of reactants. In the case of sol–gel and autocombustion using tartaric acid and urea as chelating agents, mixed phases of BiFeO3 and Bi25FeO40 were formed.
2 Experimental
2.1 Chemicals
The reagents used in the synthesis were (Fe(NO3)3·9H2O, J.T. Baker, 99.6%) (Bi(NO3)3·5H2O, Aldrich, > 98%), glycine (H2NCH2COOH, Aldrich, 99%), urea, tartaric acid (CH4N2O, C4H6O6, Alfa Aesar, 99%), and diluted nitric acid (HNO3, J.T. Baker, 70%). Figure 1 shows a diagram of the synthesis methods and the label of each sample.
2.2 Synthesis of Bismuth Ferrite Using Sol–Gel Technique
Ferric nitrate (Fe(NO3)3 9H2O, 99%) and bismuth nitrate (Bi(NO3)3·5H2O, 98%) were used as the metal salt precursors while glycine, urea, and tartaric acid were used as chelating agents and as fuels. To prepare BiFeO3 using glycine, 0.004 mol of Bi(NO3)3·5H2O and 0.004 mol of Fe(NO3)3·9H2O were dissolved in 10 mL of 5 M HNO3. This mixture was stirred for 10 min then added drop wise to 8 mmol a stirred 250-mL aqueous solution of the glycine chelating agent. The obtained solution was then vaporized at 70–80 °C under continuous magnetic stirring for 6 h until a xerogel was formed. The obtained xerogel was slightly ground and calcined in air atmosphere at 500 °C for 1 h, then, further calcined at 600 °C for another 1 h, and naturally cooled to room temperature. The synthesized BiFeO3 sample was coded G1. The same procedures were repeated to prepare BiFeO3 using the other two chelating agents, urea and tartaric acid, and were coded U1 and T1 respectively. The same procedures were also followed to synthesize BiFeO3 using higher concentrations of the starting reagents: 0.008 mol of Bi(NO3)3·5H2O, 0.008 mol of Fe(NO3)3·9H2O, and 0.016 mol of glycine; the synthesized material was coded G1H.
2.3 Synthesis of Bismuth Ferrite Using the Autocombustion Technique
Ferric nitrate and bismuth nitrate were used as the metal salt precursors. BiFeO3 nanoparticles were prepared by combustion method using three separate different organic fuels: glycine, urea, and tartaric acid. The organic materials acted as organic fuels and run as a platform for the redox reactions to arise between the reactants during the process of combustion. 0.008 mol of Bi(NO3)3·5H2O, 0.008 mol of Fe(NO3)3·9H2O, and 0.016 mol of organic fuel were mixed and put on a hotplate at 80 °C. Slurry mixtures were formed when the hygroscopic metal nitrates were mixed with the carboxylic organic fuel. The high oxygen content of the reactants and the role of the metal nitrates as oxidants make the combustion process occurred efficiently. The mixture was further heated for 10 min to initiate and achieve dehydration and the combustion process. The obtained brown powders were heated in a furnace at 500 °C in the air for 1 h, and then it was further calcined at 600 °C for another 1 h. The obtained powder samples using glycine, urea, and tartaric were coded G2, U2, and T2 respectively.
2.4 Characterization and Measurements
The phase structure, lattice constant, and crystallite size of the synthesized BiFeO3 samples were estimated via X-ray diffraction analysis (XRD, PANalytical X-ray diffraction equipment model X′Pert PRO). The optical characteristics and the band gap of the synthesized BiFeO3 samples were determined by using a double beam spectrophotometer-JASCO (model V-570 UV–Vis-NIR). The morphological structure of BiFeO3 powders was determined by using a scanning electron microscope (SEM, model Quanta 250 FEG) attached with EDX unit. The magnetic characteristics of BiFeO3 samples at room temperature were measured by using a vibrating sample magnetometer (VSM, LakeShore Model 7410). Electrical conductivity and dielectric constant properties of the prepared pellets were carried out by using LCR meter (Hitester, model Hioki 3532–50, made in Japan).
3 Results and Discussion
3.1 Phase Purity: XRD and Rietveld Refinement Analysis
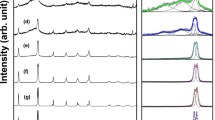
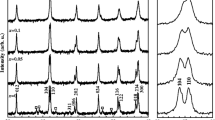
Figure 2 demonstrates the X-ray diffraction pattern of BiFeO3 powders synthesized by sol–gel and auto-combustion methods in the presence of glycine, tartaric acid, and urea. Interestingly, the synthesized powders through the sol–gel method (low concentrations of reactants, Fig. 2b) and auto-combustion (high concentrations of reactants, Fig. 2c) exhibited only the crystallographic planes of BiFeO3 without any impurities, indicating high purity phase formation. In this pattern, all the XRD planes of (012), (104), (110), (113), (006), (202), (024), (116), (122), (018), (214), (300), (208), (220), (036), (131), (312), (128), and (134) are indexed to rhombohedral structure of BiFeO3 structure (JCPDS No. 86–1518, R3c space group). The XRD pattern of BiFeO3 powder synthesized by sol–gel using high concentrations of reactants in presence of glycine (Fig. 2a) showed a very small impurity peak located at 2θ = 27.8° which is probably related to the presence of small impurity of Bi25FeO40 phase along with the basic BiFeO3 structure. These results clearly show that two reliable methods (glycine-sol–gel and glycine-autocombustion) can lead to high purity phase of BiFeO3 (Fig. 2a and c). Ortiz-Quiñonez et al. [20] reported a successful synthesis of pure BiFeO3 nanoparticles using a glycine-assisted route. However, the authors stated that the formation of the pure phase of BiFeO3 needed a calcination for the as-synthesized powder for a long period (1 h at 350 °C, 6 h at 500 °C, and 1 h at 600 °C) [20]. In the present study, the synthesized powders need only 2 h to attain a high purity BiFeO3 (1 h at 500 °C and another 1 h at 600 °C). For saving time and energy, the total calcination time was reduced from 8 [20] to 2 h only in the present work. The high flame temperature exceeds 1200 °C of glycine which results in an extremely violent combustion reaction [21]. Furthermore, the swift release of massive amounts of heat and gases may be improved the growth of the crystals and the formation of pure phase. Concerning BiFeO3 powders synthesized by sol–gel and auto-combustion methods in the presence of tartaric acid, the XRD patterns show the formation of BiFeO3 with high content of Bi25FeO40 phase reached to 44% and 38%, respectively (Fig. 2d and e). In the same context, the XRD patterns of BiFeO3 powders synthesized by sol–gel and auto-combustion methods in the presence of urea also revealed the existence of Bi25FeO40 phase with contents of 44% and 24% (Fig. 2f and g), respectively. It can be noticed that the using of the auto-combustion method yield less content of impurities compared to the sol–gel method. The formation of such bismuth oxide impurity phase (Bi25FeO40) could be explained by the tendency of the hydrated Bi(III) cations to undergo polycondensation process and forming intramolecular polycondensation species. This polycondensation process could lead to the formation of poly-nuclear cationic species: [Bi9(μ3-O)8(μ3- OH)6]5+, [Bi6O4(OH)4]6+, and [Bi6O5(OH)3]5+ [22, 23], which upon the thermal treatment could decompose into the α- and β-Bi2O3 phases. Thus, the formation of bismuth oxides (α and/or β) impurities during the synthesis of BiFeO3 could be related to the presence of these poly-nuclear moieties which largely depends on the availability of hydrated Bi(III) cations. This suggestion is further supported by our findings. In the case of glycine-assisted samples, both the combustion and the sol–gel route using a low molar concentration of the reactants led to the formation of pure BiFeO3 with no secondary phase products. This could be attributed to no presence or low number of hydrated Bi(III) cations in the combustion synthesis and the sol–gel synthesis (low concentration of reactants), respectively. On the other hand, using high concentrations of the reactants in the synthesis process resulted in the formation of more hydrated Bi(III) cations that are so enough to polycondense and produce undesirable secondary polynuclear cationic species. The presence of impurities in the case of BiFeO3 synthesized by using tartaric acid or urea, either via sol–gel or autocombustion routs, could be further explained based on the affinity of urea and tartaric acid anions to coordinate with Bi3+ and Fe3+. In contrary to glycinate ions which possess equivalent affinity constant for both Fe3+ and Bi3+ (log K = 10) [24], both the urea and tartrate anions have different coordination affinities for Bi3+ and Fe3+ [24, 25]. Consequently, the dispersion of bismuth and ferric cations in polymeric chains of the precursor solutions is not homogenous and results in creating separate α- and/or β-Bi2O3 and Bi2Fe4O9 byproducts. As a result, glycine as a chelating agent under different synthesis methods induces a pure phase formation compared to tartaric acid or urea. The least square Rietveld refinement analysis was carried out by using FullProf software to investigate the phase purity as well as to obtain the lattice parameter, unit cell volume, space group, and crystal system as shown in Fig. 3 and tabulated in Table 1. The refinement of the BiFeO3 powders synthesized using glycine-assisted route following the standard cards JPCDS No. 86–1518 revealed that both samples possess rhombohedral structure of the BiFeO3. The crystallize size of the prepared BiFeO3 samples was found by using Williamson-Hall equation:
By plotting βcosθ as a function of 4sinθ, the crystallite size was found from the intercept (D = Kλ/intercept) as shown in Fig. 4 and Table 1. The average crystallite sizes were 60.5 nm, 57.8 nm, and 46 nm for G1, G2, and G1H samples, respectively. The crystallite size is significantly influenced by the type of chelating (Table 1). The average crystallite size was 44.5, 39.7, 45.6, and 47.7 nm for T1, T2, U1, and U2 samples, respectively. The observed low crystallite size of the tartaric-assisted sample prepared using autocombustion method (T2) compared to glycine- and urea-assisted samples could be related to the combustion process occurs in different ways depending on the fuel/chelating agent. Tartaric acid has a relatively lower flame temperature (505 °C) compared to glycine and urea that have flame temperatures of 1200 °C and 600 °C respectively [21]. Subsequently, in the case of tartaric-assisted autocombustion, the rate of the combustion process is slower and the process occurs over a longer period and permits the cations to arrange more slowly within the spinel lattice. On the other hand, the more rapid release of a large volume of heat and gases through the combustion course in the case of glycine and urea favors crystallite growth. Also, the energy released from the reaction between ammonia (released from glycine or urea decomposition) and nitrogen oxides (NOx) from the decomposition of metal nitrates is much higher than that released from the reaction between oxygen and hydrocarbonaceous fragments in the case of tartaric acid [26] which also provides preferability for growth of crystallite. Figure 5 shows the polyhedral and packing structure of BiFeO3 synthesized by the glycine-assisted method.
3.2 Morphological Analysis
The SEM micrographs of the synthesized BiFeO3 powders using sol–gel and autocombustion methods in the presence of glycine, tartaric acid, and urea are shown in Fig. 6. The image of the BiFeO3 powder obtained by sol–gel method using glycine shows large irregular connected particles with clear boundary between them (Fig. 6a). The BiFeO3 powder synthesized by autocombustion using glycine exhibits irregular connected particles shape but with a smaller grain size compared to the sol–gel method (Fig. 6b). The two images reveal the presence of remarkable porous networks. The formation of such large pores is highly connected to the vast and fast release of combustion gases due to using of glycine as a chelating agent. Several reports showed that the combustion course of glycine and metal nitrates results in increasing the flame temperature to reach to 1100–1450 °C [20, 27, 28]. Such abruptly raising, in a very short time, leads to a sudden release of several gases such as CO, CO2, NH3, NO, NO2, and H2O; the coupling effect of such highly released energy and gases leads to the formation of such microporous structures. In the case of BiFeO3 powders prepared by using tartaric acid, the sol–gel method yields small size particles with non-uniform shapes related to BiFeO3 and Bi25FeO40 phases (Fig. 6c) while the autocombustion method produces smaller grains (Fig. 6d). The micrograph of the BiFeO3 prepared by sol–gel in presence of urea (Fig. 6e) has a similar morphology to that prepared by tartaric acid (Fig. 6c) while the BiFeO3 powder synthesized by the autocombustion in presence of urea (Fig. 6f) revealed the formation of elongated particles like rods (major) besides non-uniform or irregular particles (minor). The EDX spectra of the synthesized BiFeO3 powders are shown in Fig. 7. The presence of the peaks which related only to the Bi, Fe, and O elements in the EDX spectra confirms the high purity for the synthesized powders. The Bi/Fe ratio was determined by the SEM–EDX technique. These ratios were measured in two different zones of an area of about 1 μm2 each. In the case of the glycine-assisted sample G1, the experimental atomic percentages of Bi, Fe, and O in BiFeO3 are about 19.26, 20.35, and 60.4 respectively. These percentage values are very close to the theoretical values of BiFeO3 (20, 20, and 60).
3.3 Optical Properties
Figure 8 depicts the diffuse reflectance spectra for the different synthesized BiFeO3 powders within wavelengths of 200–2000 nm. Pure BiFeO3 powders prepared using glycine exhibit the lowest intensity of diffuse reflectance in the visible and near infrared spectrum, indicating high absorption ability. These nanostructures powders are the purest and the most crystalline among all the synthesized samples, so the highest crystalline order at the inter-particles contact could be responsible for such high absorption property. In contrast, the powders obtained by using tartaric acid have the highest diffuse reflectance intensity while the synthesized powders by urea have moderate intensity. A clear red shift in the absorption edge was seen for BiFeO3 prepared by glycine compared to other samples (Fig. 8). The precise band gap value of the synthesized BiFeO3 powders was measured using the Kubelka–Munk function as shown in Fig. 9. Wang et al. showed that the strong hybridization of Fe-3d, Bi-6p, and O-2p states form the top of the valence band while Fe-3d states form the bottom of the conduction band [29]. The synthesized pure BiFeO3 particles (glycine as a chelating agent) showed a visible band gap of 2.05 eV with strong long tails extending from the visible to the infrared region which is an important factor in the photocatalytic applications. The synthesized powders by using sol–gel and autocombustion methods in the presence of urea possess band gap energy of 2.17 eV and 2.14 eV, respectively. All samples showed an additional band gap energies related to indirect inter-band transition; these bands were detected in the range of 1.46–1.57 eV using the Tauc’s plot fit in the long tail-like part of the absorption spectrum, around 600 nm. Several reports demonstrated the attendance of both direct and indirect band gaps, with different energy values in BiFeO3. In these cases, the indirect transitions are situated about 0.4–1.0 eV lower than that the direct transitions [20, 30, 31]. These reports as well as our findings are consistent with some theoretical studies that predicted the presence of both indirect and direct gaps in BiFeO3 structure, and the indirect band gap is of lower energy than the direct ones [29, 32]. The calculated direct and indirect band gap values for the BiFeO3 synthesized using glycine, urea, and tartaric acid are depicted in Table 1.
3.4 Room Temperature Ferromagnetism
Figure 10 shows the room temperature ferromagnetic properties of BiFeO3 synthesized by sol–gel and autocombustion methods in the presence of glycine and urea. All samples synthesized using glycine and urea exhibit small ferromagnetic hysteresis shapes at the low applied magnetic field with paramagnetic contribution at the high magnetic field. The ferro-hysteresis behavior of the samples was established based on the presence of positive coercivity and retentivity as reported in Table 1. The magnetic properties of the BiFeO3 powders obtained by sol–gel and autocombustion from the tartaric-assisted method are shown in Fig. 11. BiFeO3 samples formed by tartaric sol–gel and autocombustion methods revealed complete room temperature ferromagnetism with saturation magnetization of 2.4 emu/g and 1.01 emu/g and coercivity (Hc) of 188.7 Oe and 57.4 Oe, respectively. Interestingly, impurities like Bi2Fe4O9 and Bi24FeO40 are paramagnetic, which do not contribute to the magnetic properties in our BiFeO3 ceramic samples. These results confirm the hypothesis that the observed ferromagnetism can be related to BiFeO3 in the powders. This behavior is consistent with previous reports of nanosized BiFeO3 particles with a mean diameter below 62 nm, whose enhanced ferromagnetism can be ascribed to the effect of breaking periodicity (62 nm) of the spin cycloid structure which is characteristic of bulk BiFeO3 [33,34,35]. Thus, the existence of apparent remanence and coercivity in the tartaric-assisted samples could be explained in terms of disturbance in the period of the spin cycloid. The particle size of BiFeO3 is lesser than the period of the incommensurate spin cycloid; thus, the antiferromagnetic order is interrupted and the spiral of BiFeO3 is partially destroyed. At the nano-size scale, the surface to volume ratio is large; thus, the surface generated uncompensated spins highly participate in the net magnetization. Wang et al. [36] have synthesized BiFeO3 nanoparticles of 60–90 nm using tartaric acid with saturation magnetization (M) value of 0.34 emu/g at 15,000 Oe which is lower than the measured value in the present work. Although BiFeO3 synthesized from urea contain the same phases as that obtained from tartaric acid, it demonstrated different magnetism behaviors. This ruled out that the Bi2Fe4O9 and Bi24FeO40 impurities are responsible for room temperature ferromagnetism. The difference in magnetic behavior may be assigned to the differences in morphology and size of the particles produced using tartaric and urea. For glycine-assisted samples, a clear ferro/antiferromagnetic behavior is established in the corresponding M–H curve (Fig. 10). The observed magnetization value at 20,000 Oe was 0.136 emu g−1 which nearly seventy times lower than that obtained using tartaric acid (2.4 emu/g). This result is consistent with XRD results and demonstrated that no undesirable secondary magnetic phases were detected. In addition, the SEM images of glycine-assisted nanocrystals demonstrated the formation of a highly porous BiFeO3 network (Fig. 6) which may induce a fractional assembly and spin cycloid discontinuation. This could result in magnetic moment dilution and consequently a significant reduction in magnetization.
3.5 Dielectric Properties
Figure 12 illustrates the change of the dielectric constant (εʹ) with frequency for BiFeO3 nanopowders synthesized by sol–gel and autocombustion methods using different chelating agents at room temperature. All the synthesized BiFeO3 powders display a decreasing trend in the dielectric constant with increasing the applied frequency in similar performance with that found for metal oxides as explained by Maxwell–Wagner model [37, 38]. Remarkably, pure single phase BiFeO3 powders synthesized by sol–gel and auto combustion methods using glycine possess room temperature dielectric constant values of 622 and 845 at a frequency of 42 Hz, respectively. In the case of BiFeO3 powders prepared using tartaric acid, the dielectric constant exhibits values of 401 and 1118 at the same frequency, respectively. The obtained BiFeO3 samples by the urea method reveal dielectric constant values of 655 and 1587. At low frequency, all types of polarization can contribute and the quick increase in the dielectric constant is essentially due to the space charge and dielectric polarization. At low frequencies, the high dielectric constant values of the synthesized BiFeO3 powders can be attributed to the influence of the relaxation polarization including space charge or orientational and also to the deformational polarization which is related to the electronic and ionic polarization [37, 38]. It is recognized that the relaxation polarization is mainly linked to the interfacial or orientational effects while the deformational polarization is related to the displacement of electrons or ions. With increasing the applied field, a decrease in the orientational polarization takes place, and accordingly the molecular dipoles require much time to change their orientation, leading to a decrease in the dielectric constant. Figure 13 displays the change of the imaginary part (dielectric loss, εʹʹ) of dielectric constant as a function of frequency for all samples. The dielectric loss shows a decreasing trend with increasing the frequency for all samples due to the decrease in the electrical resistivity [37, 38].
3.6 Electric Modulus
The analysis of complex electric modulus (M*) was used to study the frequency and temperature dependence of the conductivity of many polycrystalline oxides and ceramics materials. The complex electric modulus (M*) methodology gives a clear vision about the bulk response which can separate the local behavior of defects from the electrode effect. The complex dielectric modulus, M* (ω), can be defined as the reciprocal of the complex dielectric permittivity:
Moreover, it can also be expressed in terms of a derivative of complex impedance Z*.
where M′ and M″ are the real and imaginary parts of the complex electric modulus respectively, j = (− 1)1/2 and ω = 2πf is a radial.
Figure 14 illustrates the frequency dependence of the real part of electric modulus (M′) for BiFeO3 powders prepared by sol–gel and auto combustion using different chelating agents at room temperature. It can be seen that at low frequency, the values of M′ tend to be zero and coincide with each other, which confirms a negligibly small contribution of electrode effect [39]. With increasing the frequency, the values of M′ of all samples were strongly increased and tend to saturate at high frequency which can be assigned to the conduction phenomena due to short-range mobility of charge carriers [39]. At fixed frequency, it is also clear that the values of M′ have the highest values in the following order: G2 > G1 > U1 > T1 > U2 > T2. The change of the imaginary parts of electric modulus spectra (M″) of BiFeO3 powders prepared using sol–gel and autocombustion using different chelating agents at room temperature is shown in Fig. 15. The M″ values of the synthesized powders increase with frequency and show recurring declines and increase at high frequencies.
3.7 Electrical Conductivity
The change of the electrical conductivity with frequency for BiFeO3 powders prepared by using sol–gel and autocombustion methods using different chelating agents at room temperature is shown in Fig. 16. It can be seen that the electrical conductivity was increased with increasing the applied frequency for all BiFeO3 samples, which could be assigned to the decreases in the dielectric constant or due to the charge-hopping mechanism. The BiFeO3 powders prepared by using urea revealed the highest electrical conductivity values compared to other samples.
4 Conclusions
In this study, two dependable methods to synthesize high purity single phase BiFeO3 powders were realized. The sol–gel and autocombustion techniques using three different chelating agents, glycine, tartaric acid, and urea, were investigated. The X-ray diffraction data confirmed that the glycine-autocombustion method produced a high purity single phase of BiFeO3 using either low or high concentrations of reactants (Bi(NO3)3 + Fe(NO3)3), while the glycine-sol–gel method produced a high purity single phase of BiFeO3 using low concentrations of reactants only. In case of using tartaric acid and urea as chelating agents, mixtures of BiFeO3 and Bi25FeO40 phases were formed. The particle size, morphology, and porosity were changed by altering the chelating agent and the synthesis method. The highest purity BiFeO3 sample exhibits a visible light band gap of 2.05 eV with a long absorption tail extending to the infrared region, suggesting the suitability of this material in solar photocatalytic applications. Weak hysteresis ferromagnetic behavior was observed for glycine- and urea-assisted BiFeO3 powders with a large contribution from the paramagnetic behavior. On the other hand, tartaric-assisted samples demonstrated remarkable ferromagnetic loops with saturation magnetization reaching to 2.5 emu/g for sol–gel-assisted sample. All the synthesized samples exhibit high dielectric constant values at low frequency and follow the Maxwell–Wagner model.
References
Kuo, C.-Y., Hu, Z., Yang, J.C., Liao, S.-C., Huang, Y.L., Vasudevan, R.K., Okatan, M.B., Jesse, S., Kalinin, S.V., Li, L., Liu, H.J., Lai, C.-H., Pi, T.W., Agrestini, S., Chen, K., Ohresser, P., Tanaka, A., Tjeng, L.H., Chu, Y.H.: Single-domain multiferroic BiFeO3 films. Nat. Commun. 7, 12712 (2016)
Carranza-Celis, D., Cardona-Rodríguez, A., Narváez, J., Moscoso-Londono, O., Muraca, D., Knobel, M., Ornelas-Soto, N., Reiber, A., Ramírez, J.: Control of multiferroic properties in BiFeO3 nanoparticles. Sci. Rep. 9, 3182 (2019)
Wu, J., Fan, Z., Xiao, D., Zhu, J., Wang, J.: Multiferroic bismuth ferrite-based materials for multifunctional applications: ceramic bulks, thin films and nanostructures. Prog. Mater Sci. 84, 335–402 (2016)
Yakout, S.M.: Spintronics and innovative memory devices: a review on advances in magnetoelectric BiFeO3. J. Supercond. Novel Magn. 34, 317–338 (2021)
Bibes, M., Barthélémy, A.: Towards a magnetoelectric memory. Nat. Mater. 7, 425–426 (2008)
Spaldin, N.A., Ramesh, R.: Advances in magnetoelectric multiferroics. Nat. Mater. 18, 203–212 (2019)
Kazhugasalamoorthy, S., Jegatheesan, P., Mohandoss, R., Giridharan, N.V., Karthikeyan, B., Joseyphus, R.J., Dhanuskodi, S.: Investigations on the properties of pure and rare earth modified bismuth ferrite ceramics. J. Alloy. Compd. 493, 569–572 (2010)
Lu, J., Günther, A., Schrettle, F., Mayr, F., Krohns, S., Lunkenheimer, P., Pimenov, A., Travkin, V.D., Mukhin, A.A., Loid, A.: On the room temperature multiferroic BiFeO3: magnetic, dielectric and thermal properties. Eur. Phys. J. B 75, 451–460 (2010)
Singh, A., Kaifeng, D., Chen, J.: Temperature controlled evolution of monoclinic to super-tetragonal phase of epitaxial BiFeO3 thin films on La0.67Sr0.33MnO3 buffered SrTiO3 substrate. AIP Advances 8, 035221 (2018)
Gil-Gonzalez, E., Perejon, A., Sanchez-Jimenez, P.E., Sayagues, M.J., Raj, R., Perez-Maqueda, L.A.: Phase-pure BiFeO3 produced by reaction flash sintering of Bi2O3 and Fe2O3. J. Mater. Chem. A 6, 5356–5366 (2018)
Wang, L., Xu, J., Gao, B., Bian, L., Chen, X.: Synthesis of pure phase BiFeO3 powders by direct thermal decomposition of metal nitrates. Ceram. Int. 39, S221–S225 (2013)
Karpinsky, D.V., Eliseev, E.A., Xue, F., Silibin, M.V., Franz, A., Glinchuk, M.D., Troyanchuk, I.O., Gavrilov, S.A., Gopalan, V., Chen, L., Morozovska, A.N.: Thermodynamic potential and phase diagram for multiferroic bismuth ferrite (BiFeO3). npj Computational Materials 3, 20 (2017)
Bernardo, M.S., Jardiel, T., Peiteado, M., Caballero, A.C., Villegas, M.: Reaction pathways in the solid state synthesis of multiferroic BiFeO3. J. Eur. Ceram. Soc. 31, 3047–3053 (2011)
Zhang, Q., Sando, D., Nagarajan, V.: Chemical route derived bismuth ferrite thin films and nanomaterials. J. Mater. Chem. C 4, 4092–4124 (2016)
Selbach, S.M., Einarsrud, M., Tybell, T., Grande, T.: Synthesis of BiFeO3 by wet chemical methods. Am. Ceram. Soc. 90, 3430–3434 (2007)
Chien, S.C., Ng, D., Kumar, D., Lam, S., Jaffari, Z.H.: Investigating the effects of various synthesis routes on morphological, optical, photoelectrochemical and photocatalytic properties of single-phase perovskite BiFeO3. J. Phys. Chem. Solids 160, 110342 (2022)
Lam, S., Jaffari Z.H., Sin, J., Zeng, H., Lin, H., Li, H., Mohamed, A.R., Ng, D.: Surface decorated coral-like magnetic BiFeO3 with Au nanoparticles for effective sunlight photodegradation of 2,4-D and E. coli inactivation. Journal of Molecular Liquids 326, 115372 (2021)
Lam, S., Jaffari, Z.H., Sin, J., Zeng, H., Lin, H., Li, H., Mohamed, A.R.: Insight into the influence of noble metal decorated on BiFeO3 for 2, 4-dichlorophenol and real herbicide wastewater treatment under visible light. Colloids Surf., A 614, 126138 (2021)
Jaffari, Z.H., Lam, S., Sin, J., Zeng, H., Mohamed, A.R.: Magnetically recoverable Pd-loaded BiFeO3 microcomposite with enhanced visible light photocatalytic performance for pollutant, bacterial and fungal elimination. Sep. Purif. Technol. 236, 116195 (2020)
Ortiz-Quiñonez, J.L., Díaz, D., Zumeta-Dubé, I., Arriola-Santamaría, H., Betancourt, I., Santiago-Jacinto, P., Nava-Etzana, N.: Easy synthesis of high-purity BiFeO3 nanoparticles: new insights derived from the structural, optical, and magnetic characterization. Inorg. Chem. 52, 10306–10317 (2013)
Dumitrescu, A.M., Samoila, P.M., Nica, V., Doroftei, F., Iordan, A.R., Palamaru, M.N.: Study of the chelating/fuel agents influence on NiFe2O4 samples with potential catalytic properties. Powder Technol. 243, 9–17 (2013)
Thurston, J.H., Swenson, D.C., Messerle, L.: Solvolytic routes to new nonabismuth hydroxy- and alkoxy-oxo complexes: synthesis, characterization and solid-state structures of novel nonabismuth polyoxo cations Bi9(m3-O)8(m3-OR)65+ (R5 H, Et). Chem. Commun. 33, 4228–4230 (2005)
Miersch, L., Schlesinger, M., Troff, R.W., Schalley, C.A., Rffer, T., Lang, H., Zahn, D., Mehring, M.: Hydrolysis of a basic bismuth nitrate—formation and stability of novel bismuth oxido Clusters. Chem. Eur. J. 17, 6985–6990 (2011)
Martell, A.E., Smith, R. M.: Critical Stability Constants. Springer (1989)
Hiroshi, Y., Tsuyoshi, M., Yasuyoshi, M., Satoshi, K.: Complex formation between iron (III) and tartaric and citric acids in a wide pH range 1 to 13 as studied by magnetic susceptibility measurements. Chem. Lett. 23, 281–284 (1994)
Hwang, C., Tsai, J., Huang, T.-H.: Combustion synthesis of Ni–Zn ferrite by using glycine and metal nitrates—investigations of precursor homogeneity, product reproducibility, and reaction mechanism. Mater. Chem. Phys. 93, 330–336 (2005)
Valefi, M., Falamaki, C., Ebadzadeh, T., Hashji, M.S.: New insights of the glycine-nitrate process for the synthesis of nano-crystalline 8YSZ. J. Am. Ceram. Soc. 90, 2008–2014 (2007)
Hwang, C., Wu, T., Wan, J., Tsai, J.: Development of a novel combustion synthesis method for synthesizing of ceramic oxide powders. Mater. Sci. Eng., B 111, 49–56 (2004)
Wang, H., Zheng, Y., Cai, M., Huang, H., Chan, H.L.W.: First-principles study on the electronic and optical properties of BiFeO3. Solid State Commun. 149, 641–644 (2009)
Fruth, V., Tenea, E., Gartner, M., Anastasescu, M., Berger, D., Ramer, R., Zaharescu, M.: Preparation of BiFeO3 films by wet chemical method and their characterization. J. Eur. Ceram. Soc. 27, 937–940 (2007)
Lam, S.-M., Sin, J.-C., Mohamed, A.R.: A newly emerging visible light-responsive BiFeO3 perovskite for photocatalytic applications: a mini review. Mater. Res. Bull. 90, 15–30 (2017)
McDonnell, K.A., Wadnerkar, N., English, N.J., Rahman, M., Dowling, D.: Photo-active and optical properties of bismuth ferrite (BiFeO3): an experimental and theoretical study. Chem. Phys. Lett. 572, 78–84 (2013)
Huang, F., Wang, Z., Lu, X., Zhang, J., Min, K., Lin, W., Ti, R., Xu, T.T., He, J., Yue, C., Zhu, J.: Peculiar magnetism of BiFeO3 nanoparticles with size approaching the period of the spiral spin structure. Sci. Rep. 3, 2907 (2013)
Castillo, M.E., Shvartsman, V.V., Gobeljic, D., Gao, Y., Landers, J., Wende, H., Lupascu, D.C.: Effect of particle size on ferroelectric and magnetic properties of BiFeO3 nanopowders. Nanotechnology 24, 355701 (2013)
Reddy, V.A., Pathak, N.P., Nath, R.: Particle size dependent magnetic properties and phase transitions in multiferroic BiFeO3 nano-particles. J. Alloy. Compd. 543, 206–212 (2012)
Wang, X., Zhang, Y., Wu, Z.: Magnetic and optical properties of multiferroic bismuth ferrite nanoparticles by tartaric acid-assisted sol–gel strategy. Mater. Lett. 64, 486–488 (2010)
Ahmad, N., Khan, S., Ansari, M.M.N.: Optical, dielectric and magnetic properties of Mn doped SnO2 diluted magnetic semiconductors. Ceram. Int. 44, 15972–15980 (2018)
Parveen, A., Ahmad, S.A., Agrawal, S., Azam, A.: Room temperature variation in dielectric and electrical properties of Mn doped SnO2 nanoparticles. Materials Today: Proceedings 4, 9429–9433 (2017)
Wang, Y., Pu, Y., Wang, Z., Li, X., Cui, Y.: Dielectric, modulus and impedance analysis of (Ba0.9Bi0.1)(Ti0.9Al0.1)O3 ceramics. J Mater Sci: Mater Electron 28, 4245–4252 (2017)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors gratefully acknowledge the funds for this work provided by Project No. 10070102, Inorganic Chemistry Department, National Research Center, Egypt.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wahba, M.A., Yakout, S.M., Youssef, A.M. et al. Chelating Agents Assisted Rapid Synthesis of High Purity BiFeO3: Remarkable Optical, Electrical, and Magnetic Characteristics. J Supercond Nov Magn 35, 3689–3704 (2022). https://doi.org/10.1007/s10948-022-06425-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-022-06425-z