Abstract
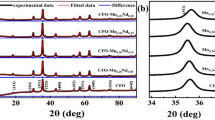
Magnetic nanoparticles of ferromagnetic and antiferromagnetic phases were synthesized from controlled heat treatment of dry ferritin powder in a bottom-up approach. Heat treatment paves the way for synthesis of Fe2O3 solid phase from iron molecular complexes stored in the cavity of ferritin; the strong increase (~ 150 times) in saturation magnetization is a sign of the presence of ferromagnetic exchange coupling which exists only in solid phase. In this experimental study, vibrating sample magnetometer (VSM) was used to study magnetic induction. X-ray diffraction (XRD) and scanning electron microscopy (SEM) were employed for analysis of structure and morphology of the samples. Differential scanning calorimetry (DSC) was used to search for indicators of structural phase transformation through scanning of temperature. The nanoparticle size with highly narrow distribution (mean size ~ 6 nm) was observed by SEM for sample annealed at 430 °C. As indicated by XRD measurements, the majority phase of Fe2O3 nanoparticles was amorphous up to 500 °C, whereas DSC demarcates the crystalline phase transition temperature at 545 °C. The annealing temperature range from 400 to 500 °C was found to be suitable for growing ferromagnetic nanoparticles endowed with high saturation magnetization and low coercivity. At higher range of annealing temperature (500–700 °C), XRD confirms the presence of α-Fe2O3 (haematite) phase which is an antiferromagnetic crystalline system with weak magnetization. A systematic decline of magnetization on increasing the annealing temperature beyond 500 °C was attributed to finite size effects and increased purity of antiferromagnetic phase.





Similar content being viewed by others
References
MacHala, L., Tuček, J., Zbořil, R.: Polymorphous transformations of nanometric iron(III) oxide: a review. Chem. Mater. 23, 3255–3272 (2011). https://doi.org/10.1021/cm200397g
Silveyra, M.J., Ferrara, E., Huber, D.I., Monson, T.C.: Sustainable and electrified world. Science (80-). 362, 1–9 (2018). https://doi.org/10.1126/science.aao0195
Behrens, S., Appel, I.: ScienceDirect Magnetic nanocomposites. Curr. Opin. Biotechnol. 39, 89–96 (2016). https://doi.org/10.1016/j.copbio.2016.02.005
Lee, H., Shin, T.H., Cheon, J., Weissleder, R.: Recent developments in magnetic diagnostic systems. Chem. Rev. 115, 10690–10724 (2015). https://doi.org/10.1021/cr500698d
Tietze, R., Zaloga, J., Unterweger, H., Lyer, S., Friedrich, R.P., Janko, C., Pöttler, M., Dürr, S., Alexiou, C.: Magnetic nanoparticle-based drug delivery for cancer therapy. Biochem. Biophys. Res. Commun. 468, 463–470 (2015). https://doi.org/10.1016/j.bbrc.2015.08.022
Hauser, A.K., Wydra, R.J., Stocke, N.A., Anderson, K.W., Hilt, J.Z.: Magnetic nanoparticles and nanocomposites for remote controlled therapies. J. Control. Release. 219, 76–94 (2015). https://doi.org/10.1016/j.jconrel.2015.09.039
Odenbach, S.: Ferrofluids and their applications. MRS Bull. 38, 921–924 (2013). https://doi.org/10.1557/mrs.2013.232
Gawande, M.B., Monga, Y., Zboril, R., Sharma, R.K.: Silica-decorated magnetic nanocomposites for catalytic applications. Coord. Chem. Rev. 288, 118–143 (2015). https://doi.org/10.1016/j.ccr.2015.01.001
Wen, T., Krishnan, K.M.: Cobalt-based magnetic nanocomposites: fabrication, fundamentals and applications. J. Phys. D. Appl. Phys. 44, 1–24 (2011). https://doi.org/10.1088/0022-3727/44/39/393001
Viswanathan, V., Laha, T., Balani, K., Agarwal, A., Seal, S.: Challenges and advances in nanocomposite processing techniques. Mater. Sci. Eng. R. Rep. 54, 121–285 (2006). https://doi.org/10.1016/j.mser.2006.11.002
Zeng, H., Li, J., Wang, Z.L., Liu, J.P., Sun, S.: Bimagnetic core/shell FePt/Fe3O4 nanoparticles. Nano Lett. 4, 187–190 (2004). https://doi.org/10.1021/nl035004r
Icten, O., Hosmane, N.S., Kose, D.A., Zumreoglu-Karan, B.: Magnetic nanocomposites of boron and vitamin C. New J. Chem. 41, 3646–3652 (2017). https://doi.org/10.1039/c6nj03894h
Lu, Y., Yin, Y., Mayers, B.T., Xia, Y.: Modifying the surface properties of superparamagnetic iron oxide nanoparticles through a sol-gel approach. Nano Lett. 2, 183–186 (2002). https://doi.org/10.1021/nl015681q
Cao, D., Li, H., Pan, L., Li, J., Wang, X., Jing, P., Cheng, X., Wang, W., Wang, J., Liu, Q.: High saturation magnetization of γ 3-Fe 2 O 3 nano-particles by a facile one-step synthesis approach. Sci. Rep. 6, 1–9 (2016). https://doi.org/10.1038/srep32360
Gupta, A.K., Gupta, M.: Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 26, 3995–4021 (2005). https://doi.org/10.1016/j.biomaterials.2004.10.012
Ohno, K., Mori, C., Akashi, T., Yoshida, S., Tago, Y., Tsujii, Y., Tabata, Y.: Fabrication of contrast agents for magnetic resonance imaging from polymer-brush-afforded iron oxide magnetic nanoparticles prepared by surface-initiated living radical polymerization. Biomacromolecules. 14, 3453–3462 (2013). https://doi.org/10.1021/bm400770n
Conde, J., Doria, G., Baptista, P.: Noble metal nanoparticles applications in cancer. J. Drug Deliv. 2012, 1–12 (2012). https://doi.org/10.1155/2012/751075
Kumar, P., Rawat, N., Hang, D.R., Lee, H.N., Kumar, R.: Controlling band gap and refractive index in dopant-free α-Fe2O3 films. Electron. Mater. Lett. 11, 13–23 (2015). https://doi.org/10.1007/s13391-014-4002-0
Kumar, P., Kumar, R., Lee, H.N.: Magnetic field induced one-dimensional nano/micro structures growth on the surface of iron oxide thin film. Thin Solid Films. 592, 155–161 (2015). https://doi.org/10.1016/j.tsf.2015.08.047
Patil, P.R., Krishnamurthy, V.N., Joshi, S.S.: Differential scanning calorimetric study of HTPB based composite propellants in presence of nano ferric oxide. Propellants, Explos. Pyrotech. 31, 442–446 (2006). https://doi.org/10.1002/prep.200600059
MacHala, L., Zboril, R., Gedanken, A.: Amorphous iron (III) oxide-a review. J. Phys. Chem. B. 111, 4003–4018 (2007). https://doi.org/10.1021/jp064992s
Jungwirth, T., Marti, X., Wadley, P., Wunderlich, J.: Antiferromegnetic Spintronics. Nat. Nanotechnol. 11, 231–241 (2016). https://doi.org/10.1038/nnano.2016.18
Bhowmik, R.N., Sarvanan, A.: Surface magnetism, Morin transition, and magnetic dynamics in antiferromagnetic α-Fe2O3 (hematite) nanograins. J. Appl. Phys. 107, 053916–1–10 (2010). https://doi.org/10.1063/1.3327433
Zeng, Q., Reuther, R., Oxsher, J., Wang, Q.: Characterization of horse spleen apoferritin reactive lysines by MALDI-TOF mass spectrometry combined with enzymatic digestion. Bioorg. Chem. 36, 255–260 (2008). https://doi.org/10.1016/j.bioorg.2008.06.001
He, D., Marles-Wright, J.: Ferritin family proteins and their use in bionanotechnology. New Biotechnol. 32, 651–657 (2015). https://doi.org/10.1016/j.nbt.2014.12.006
Kim, J.W., Choi, S.H., Lillehei, P.T., Chu, S.H., King, G.C., Watt, G.D.: Electrochemically controlled reconstitution of immobilized ferritins for bioelectronic applications. J. Electroanal. Chem. 601, 8–16 (2007). https://doi.org/10.1016/j.jelechem.2006.10.018
Park, C.W., Park, H.J., Kim, J.H., Won, K., Yoon, H.H.: Immobilization and characterization of ferritin on gold electrode. Ultramicroscopy. 109, 1001–1005 (2009). https://doi.org/10.1016/j.ultramic.2009.03.002
Jutz, G., Van Rijn, P., Santos Miranda, B., Böker, A.: Ferritin: a versatile building block for bionanotechnology. Chem. Rev. 115, 1653–1701 (2015). https://doi.org/10.1021/cr400011b
Volatron, J., Carn, F., Kolosnjaj-Tabi, J., Javed, Y., Vuong, Q.L., Gossuin, Y., Ménager, C., Luciani, N., Charron, G., Hémadi, M., Alloyeau, D., Gazeau, F.: Ferritin protein regulates the degradation of iron oxide nanoparticles. Small. 13, 1–13 (2017). https://doi.org/10.1002/smll.201602030
Chasteen, N.D., Harrison, P.M.: Mineralization in ferritin- an efficient means of iron storage. J. Struct. Biol. 126, 182–194 (1999). https://doi.org/10.1006/jsbi.1999.4118
Qu, X., Kobayashi, N., Komatsu, T.: Solid nanotubes comprising α-Fe2O3 nanoparticles prepared from ferritin protein. ACS Nano. 4, 1732–1738 (2010). https://doi.org/10.1021/nn901879d
Singh, A., Mukherjee, M.: Analysis of polypeptide inter-chain entanglements using swelling dynamics of a spin coated protein layer. Thin Solid Films. 691(1–6), 137605 (2019). https://doi.org/10.1016/j.tsf.2019.137605
Singh, A., Konovalov, O., Novak, J., Vorobiev, A.: The sequential growth mechanism of a protein monolayer at the air-water interface. Soft Matter. 6, 3826–3831 (2010). https://doi.org/10.1039/b925365c
Singh, A., Konovalov, O.: Measuring elastic properties of a protein monolayer at water surface by lateral compression. Soft Matter. 9, 2845–2851 (2013). https://doi.org/10.1039/c2sm26410b
Bean, C.P., Livingston, J.D.: Superparamagnetism. J. Appl. Phys. 30, S120–S129 (1959). https://doi.org/10.1063/1.2185850
Kittel, C.: Introduction to Solid State Physics. John Wiley & Sons, Delhi (1999)
García-Prieto, A., Alonso, J., Muñoz, D., Marcano, L., Abad Díaz De Cerio, A., Fernández De Luis, R., Orue, I., Mathon, O., Muela, A., Fdez-Gubieda, M.L.: On the mineral core of ferritin-like proteins: structural and magnetic characterization. Nanoscale. 8, 1088–1099 (2016). https://doi.org/10.1039/c5nr04446d
St. Pierre, T.G., Chan, P., Bauchspiess, K.R., Webb, J., Betteridge, S., Walton, S., Dickson, D.P.E.: Synthesis, structure and magnetic properties of ferritin cores with varying composition and degrees of structural order: models for iron oxide deposits in iron-overload diseases. Coord. Chem. Rev. 151, 125–143 (1996). https://doi.org/10.1016/s0010-8545(96)90201-5
Patterson, A.L.: The Scherrer formula for X-ray particle size determination. Phys. Rev. 56, 978–982 (1939). https://doi.org/10.1103/PhysRev.56.978
Descamps, M., Dudognon, E.: Crystallization from the amorphous state: nucleation-growth, decoupling, polymorphism, the role of interfaces. J. Pharm. Sci. 103, 2615–2628 (2014). https://doi.org/10.1002/jps.24016
Russo, C.J., Passmore, L.A.: Electron microscopy, ultrastable gold substrates for electron cryomicroscopy. Science (80-). 346, 1377–1380 (2014). https://doi.org/10.1126/science.1259530
Coey, J.M.D.: Magnetism in amorphous solids. Physics of solids and liquids. Springer, Boston, MA (1985). https://doi.org/10.1007/978-1-4757-9156-3_13
Frandsen, C., Mørup, S.: Spin rotation in α-Fe2O3 nanoparticles by interparticle interactions. Phys. Rev. Lett. 94(1–4), 027202 (2005). https://doi.org/10.1103/PhysRevLett.94.027202
Kodama, R.H., Berkowitz, A.E., McNiff, E.J.J., Foner, S.: Surface spin disorder in NiFe2O4 nanoparticles. Phys. Rev. Lett. 77, 394–397 (1996). https://doi.org/10.1103/PhysRevLett.77.394
Ali, A., Zafar, H., Zia, M., ul Haq, I., Phull, A.R., Ali, J.S., Hussain, A.: Synthesis, characterization, applications and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 9, 49–67 (2016). https://doi.org/10.2147/NSA.S99986
Akbarzadeh, A., Samiei, M., Davaran, S.: Magnetic nanoparticles: preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 7, 1–13 (2012). https://doi.org/10.1186/1556-276X-7-144
Acknowledgements
SK thankfully acknowledges the University Grants Commission for National Fellowship scheme for SC (erstwhile–RGNF, Lett. No. F1-17.1/2016-17/RGNF-2015-17-SC-HIM-18502/(SA-III/Website)) for the financial support as fellowship towards pursuance of Ph.D. We acknowledge XRD facility established under DST-SAIF at the Panjab University. We acknowledge the DSC facility under DST-CURIE grant (SR/CURIE-PHASE-III/01/2015 (G)) at the Banasthali Vidyapith (DSC 204 F1 Phoenix, NETZSCH) and IIT Mandi for SEM facility (JFEI, Nova Nano SEM-450). Authors are thankful to Varsha for conducting measurements at the Banasthali Vidyapith.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, S., Thakur, A., Gupta, S.K. et al. A Facile Route to Synthesis of Ferromagnetic and Antiferromagnetic Phases of Iron Oxide Nanoparticles by Controlled Heat Treatment of Ferritin. J Supercond Nov Magn 33, 3841–3852 (2020). https://doi.org/10.1007/s10948-020-05649-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-020-05649-1