Abstract
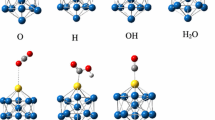
It is suggested that a set of discrete Cu nanoclusters satisfying the conditions of structural and electron stability should be used as models of active sites on supported metal catalysts. The close-packed Cu20 tetrahedral nanocluster that satisfies these two conditions was taken as a base model of active sites on supported copper catalysts. Theoretical analysis of two possible mechanisms of C-Cl bond dissociation of 1,2-dichloroethane on copper catalysts was performed by the density functional theory method. The first mechanism involves sequential splitting of C-Cl bonds in the molecule in three stages with further stabilization of chloroalkyl intermediates (stepwise mechanism). All these stages are activated. The limiting stage is the one that corresponds to dissociation of the first C-Cl bond with an activation energy of E# = 34.3 kcal/mol. The second mechanism corresponds to the simultaneous elimination of two chlorine atoms from 1,2-dichloroethane with liberation of ethylene in the gas phase; this is a one-stage process with an activation energy of E# = 26.1 kcal/mol (direct mechanism). A comparison of the two reaction routes shows that the direct mechanism is most probable on copper catalysts.
Similar content being viewed by others
References
G. M. Bickle, T. Suzuki, and Y. Mitarai, Process Safety Environ. Prot., 70, 40 (1992).
K. E. Nelson, Industrial Environmental Chemistry, D. T. Sawyer and A. E. Martell (eds.), Plenum, New York (1992).
A. Maccol, Chem. Rev., 69, 33–60 (1969).
R. M. Lago, H. L. Greene, S. C. Tsang, and M. Odlyha, Appl. Catal. B, 8, 107–121 (1996).
J. R. Gonzalez-Velasco, R. Lopez-Fonseca, A. Aranzabal, et al., ibid., 24, 233–242 (2000).
A. Aranzabal, J. A. Gonzalez-Marcos, R. Lopez-Fonseca, et al., Stud. Surf. Sci. Catal. B, 130, 1229–1234 (2000).
L. N. Zanaveskin, V. A. Aver’yanov, and Yu. A. Treger, Usp. Khim., 65, 667–675 (1996).
S. T. Ceyer, Ann. Rev. Phys. Chem., 39, 479–510 (1988).
J. L. Lin and B. E. Bent, J. Phys. Chem., 96, 8529–8538 (1992).
R. G. Parr and W. Yang, Density-Functional Theory of Atoms and Molecules, Oxford University Press, New York (1989).
A. D. Becke, Phys. Rev., A33, 2786–2797 (1986).
A. D. Becke, J. Chem. Phys., 98, 5648–5652 (1993).
C. Lee, W. Yang, and R. G. Parr, Phys. Rev., B37, 785–797 (1988).
W. Stevens, H. Bash, and J. Krauss, J. Chem. Phys., 81, 6026–6035 (1984).
T. R. Cundari and W. J. Stevens, ibid., 98, 5555–5567 (1993).
R. Krishnan, J. S. Seger, and J. A. Pople, ibid., 72, 650–661 (1980).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, et al., GAUSSIAN, Revision A.11, Pittsburgh, PA (2001).
C. Winkler, A. Carew, R. Raval, et al., Surf. Rev. Lett., 8, 693–697 (2001).
S. Haq, C. Winkler, A. Carew, et al., J. Catal., 226, 1–8 (2004).
D. G. Leopold, J. Ho, and W. C. Lineberger, J. Chem. Phys., 86, 1715–1726 (1987).
C. L. Pettiette, S. H. Yang, M. J. Craycraft, et al., ibid., 88, 5377–5388 (1988).
O. Cheshnovsky, K. J. Taylor, J. Conceicao, and R. E. Smalley, Phys. Rev. Lett., 64, 1785–1788 (1990).
K. J. Taylor, C. L. Pettiette, O. Cheshnovsky, and R. E. Smalley, J. Chem. Phys., 96, 3319–3327 (1992).
K. A. Jackson, Phys. Rev. B, 47, 9715–9722 (1993).
C. Massobrio, A. Pasquarello, and A. J. Dal Corso, Chem. Phys., 109, 6626–6630 (1998).
K. Jug, B. Zimmermann, P. Calaminici, and A. M. Koster, ibid., 116, 4497–4507 (2002).
N. Lopez, F. Illas, and G. Pacchioni, J. Am. Chem. Soc., 121, 813–821 (1999).
W. D. Knight, K. Clemenger, W. A. de Heer, et al., Phys. Rev. Lett., 52, 2141–2143 (1984).
K. H. Meiwes Broer, in: Metal Clusters at Surfaces, K. H. Meiwes Broer (ed.), Springer, Berlin (2000), p. 151.
W. A. de Heer, Rev. Mod. Phys., 65, 611–676 (1993).
M. Kabir, A. Mookerjee, and A. K. Bhattacharya, Eur. Phys. J. D: Atomic, Molecular and Optical Physics, 31, 477–485 (2004).
S. N. Khanna and P. Jena, Phys. Rev. Lett., 69, 1664–1667 (1992).
H. S. Taylor, Rroc. R. Soc. London, Ser. A, 108, 105–112 (1925).
T. Zambelli, J. Wintterlin, J. Trost, and G. Ertl, Science, 273, 1688–1696 (1996).
S. Dahl, A. Logadottir, R. C. Egeberg, et al., Phys. Rev. Lett., 83, 1814–1817 (1999).
A. S. Y. Chan, S. Turton, and R. G. Jones, Surf. Sci., 433-435, 234–238 (1999).
W. K. Walter and R. G. Jones, ibid., 264, 391–405 (1992).
M. Kerkar, W. K. Walter, D. P. Woodruff, et al., ibid., 268, 36–44 (1992).
S. Turton, M. Kadodwava, and R. G. Jones, ibid., 442, 517–530 (1999).
S. Turton and R. G. Jones, ibid., 468, 165–175 (2000).
A. S. Y. Chan and R. G. Jones, J. Vac. Sci. Techn. A, 19, 1474–1488 (2001).
R. G. Jones, A. S. Chan, S. Turton, et al., J. Phys. Chem. B, 105, 10600–10609 (2001).
V. I. Avdeev, V. I. Kovalchuk, G. M. Zhidomirov, and J. L. d’Itri, Surf. Sci., 583, 46–59 (2005).
W. K. Walter, R. G. Jones, K. C. Waugh, and S. Bailey, Catal. Lett., 24, 333–342 (1999).
Y. Anju, I. Mochida, H. Yamamoto, et al., Bull. Chem. Soc. Jpn., 45, 2319–2323 (1972).
M. X. Yang, S. Sarkar, B. E. Bent, et al., Langmuir, 13, 229–242 (1997).
Author information
Authors and Affiliations
Corresponding author
Additional information
__________
Translated from Zhurnal Strukturnoi Khimii, Vol. 48, Supplement, pp. S169–S179, 2007.
Original Russian Text Copyright © 2007 by V. I. Avdeev, V. I. Kovalchuk, G. M. Zhidomirov, and J. L. d’Itri
Rights and permissions
About this article
Cite this article
Avdeev, V.I., Kovalchuk, V.I., Zhidomirov, G.M. et al. Models of active sites in supported Cu metal catalysts in 1,2-dichloroethane dechlorination. DFT analysis. J Struct Chem 48 (Suppl 1), S160–S170 (2007). https://doi.org/10.1007/s10947-007-0159-9
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10947-007-0159-9