Abstract
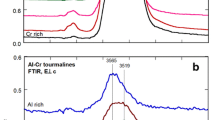
EPR and optical absorption spectra of Cu2+ ion were investigated in natural elbaites from Brazil and Zambia and in synthetic olenite single crystal. In elbaite from Zambia, the content of Cu2+ ions was found to be about 0.006 pfu, whereas in Brazilian elbaite the amount of this ion can approach up to 0.2 pfu. The rose color of elbaite from Zambia is mainly due to optical absorption at 515 nm related to Mn3+ ions. The blue color of Brazilian elbaite is related to Cu2+ absorption bands at 695 nm and 920 nm. Spin Hamiltonian parameters of Cu2+ calculated from the angular dependence of the EPR spectra are: g x = 2.054, g y = 2.092, g z = 2.374; A x = 27.8·10−4 cm−1, A y = 59.3·10−4 cm− 1, A z = 133.2·10−4 cm−1. We propose that Cu2+ ions enter into Y octahedra with common edges; the symmetry of these Y octahedra is lowered because of local disorder induced by occupancy of the Y site by cations of very different size and charge, such as Li+, Al3+, and Cu2+.
Similar content being viewed by others
References
A. Abragam and B. Bleaney, Electron Paramagnetic Resonance of Transition Ions, Clarendon Press, Oxford (1970).
S. A. Altschuler and B. M. Kozyrev, Electron Paramagnetic Resonance of Compounds with Elements of Transition Groups [in Russian], Nauka, Moscow (1972).
M. J. Buerger, C. W. Burnham, and D. R. Peacor, Acta Cryst. 15, 583–590 (1962).
P. C. Burns, D. J. MacDonald, and F. C. Hawthorne, Can. Mineral., 32, 31–34 (1994).
U. Henn, H. Bank, F. H. Bank, H. von Platen, and W. Hofmeister, Mineral. Mag., 54, 553–557 (1990).
K. Krambrock, M. V. B. Pinheiro, K. J. Guedes, et al., Phys. Chem. Miner., 31, 168–175 (2004a).
K. Krambrock, S. M. Medeiros, K. J. Guedes, et al., in: “Applied Mineralogy: Developments in Science and Technology,” Pecchio et al. (ed.), Vol. 2, M. International Council for Applied Mineralogy do Brasil, São Paulo, 567–570 (2004b).
D. J. MacDonald and F. C. Hawthorne, Acta Crystallogr., C51, 555–557 (1995).
P. G. Manning, Can. Mineral., 9, 678–690 (1969).
A. D. Rae, J. Chem. Phys., 50, 2672–2685 (1969).
G. R. Rossman, E. Fritsch, and J. E. Shigley, Am. Mineral., 76, 1479–1484 (1991).
Z. V. Bershov, V. O. Martirosyan, A. S. Marfunin, et al., Kristallogr., 13, 730–732 (1968).
I. Petrov, Am. Miner., 75, 237–239 (1990).
A. S. Lebedev, S. V. Kargal’tsev, and V. S. Pavlyuchenko, Materials on Geneticand Experimental Mineralogy. Crystal Growth and Properties [in Russian], Nauka, Novosibirsk (1988).
R. I. Mashkovtsev, A. S. Lebedev, and N. A. Balakireva, Zh. Prikl. Spektroskop., 52, 311–314 (1990).
Author information
Authors and Affiliations
Additional information
Original Russian Text Copyright © 2006 by R. I. Mashkovtsev, S. Z. Smirnov, and J. E. Shigley
__________
Translated from Zhurnal Strukturnoi Khimii, Vol. 47, No. 2, pp. 259–263, March–April, 2006.
Rights and permissions
About this article
Cite this article
Mashkovtsev, R.I., Smirnov, S.Z. & Shigley, J.E. The features of the Cu2+-entry into the structure of tourmaline. J Struct Chem 47, 252–257 (2006). https://doi.org/10.1007/s10947-006-0294-8
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10947-006-0294-8