Abstract
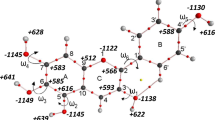
The electronic structure of HOCN, HSCN, HNCO, and HNCS molecules and [OCN]− and [SCN]− anions has been studied by ab initio calculations at HF/6-31G(d), HF/6-31G(d, p), MP2/6-31G(d)//HF/6-31G(d), and MP2/6-31G(d, p)//HF/6-31G(d, p) levels of theory. The HNCO and HNCS molecules are shown to have higher thermodynamic stability than HOCN and HSCN, respectively. The protolyte strength series are substantiated: HSCN > HOCN, HNCS > HNCO, HOCN > HNCO, HSCN > HNCS. Computations including electron correlation [MP2/6-31G(d)//HF/6-31G(d) and MP2/6-31G(d, p)//HF/6-31G (d, p)] reproduce the general sequence of proton-donor properties: HSCN > HOCN > HNCS > HNCO, which coincides with the hydrophobicity series for the compounds. The relative proton-donor capacity of these acids in water solutions is generally governed by the electronic structure and by the size of their molecules and [OCN]− and [SCN]− anions, but not by medium effects.
Similar content being viewed by others
References
K. Saito, S. Hayakawa, F. Takei, and H. Yamadera, in: Chemistry and Periodic Table [Russian translation], K. Saito (ed.), Mir, Moscow (1982).
A. Wells, Structural Inorganic Chemistry, Vol. 3, Oxford University Press, New York (1983).
A. M. Golub, H. Keller, V. V. Skopenko, et al., in: Pseudohalide Chemistry [Russian translation], A. M. Golub, H. Keller, V. V. Skopenko (eds.), Vishchaya Shkola, Kiev (1981).
J. H. Teles, G. Maier, B. A. Hess, et al., Chem. Ber., 122, No. 4, 753–766 (1989).
N. S. Akhmetov, General and Inorganic Chemistry [in Russian], Vysshaya Shkola, Academia, Moscow (2001).
J. H. Boughton and R. N. Keller, J. Inorg. Nucl. Chem., 28, No. 12, 2851–2859 (1966).
W. J. Hehre, L. Radom, P. v. R. Schleyer, and J. A. Pople, Ab Initio Molecular Orbital Theory, Wiley, New York (1986).
J. Dennis and R. Schnabel, Numerical Methods for Unconstrained Optimization and Nonlinear Equations, Prentice Hall, Englewood Cliffs, NJ (1983).
K. S. Krasnov, N. V. Filippenko, V. A. Bobkova, et al., Molecular Constants of Inorganic Compounds: Reference Book [in Russian], K. S. Krasnov (ed.), Khimiya, Leningrad (1979).
L. V. Gurvich, G. V. Karachevtsev, V. N. Kondratiev, et al., Chemical Bond Cleavage Energies. Ionization Potentials and Electron Affinities [in Russian], V. N. Kondratiev (ed.), Nauka, Moscow (1974).
M. I. Kabachnik, Usp. Khim., 48, No. 9, 1523–1547 (1979).
R. De Kock and C. Jasperse, Inorg. Chem., 22, No. 26, 3839–3843 (1983).
S. Olivella, F. Urpi, and J. Vilarrasa, J. Comput. Chem., 5, No. 3, 230–236 (1984).
J. L. Ozment and A. M. Schmiedekamp, Int. J. Quant. Chem., 43, 783–800 (1992).
I. H. Williams, G. M. Maggiora, and R. L. Schowen, J. Am. Chem. Soc., 102, No. 27, 7831–7839 (1980).
W. Hasel, T. F. Hendrickson, and W. C. Still, Tetrahedron Comp. Method., 1, No. 2, 103–116 (1988).
W. C. Still, A. Tempczyk, R. C. Hawley, and Th. Hendrickson, J. Am. Chem. Soc., 112, No. 16, 6127–6129 (1990).
N. Bodor, Z. Gabanyi, and Wong Chu-Kuok, ibid., 111, No. 11, 3783–3786 (1989).
A. Gavezotti, ibid., 105, No. 16, 5220–5225 (1983).
A. K. Ghose and G. M. Crippen, J. Chem. Inf. Comput. Sci., 27, No. 1, 21–35 (1987).
V. N. Viswanadhan, A. K. Ghose, G. N. Revankar, and R. K. Robins, ibid., 29, No. 3, 163–172 (1989).
K. J. Miller, J. Am. Chem. Soc., 112, No. 23, 8533–8542 (1990).
A. K. Ghose and G. M. Crippen, J. Comput. Chem., 7, No. 4, 565–577 (1986).
A. K. Ghose, A. Pritchett, and G. M. Crippen, ibid., 99, No. 1, 80–90 (1988).
Author information
Authors and Affiliations
Additional information
Original Russian Text Copyright © 2005 A. N. Pankratov and S. S. Khmelev
__________
Translated from Zhurnal Strukturnoi Khimii, Vol. 46, No. 3, pp. 416–421, May–June, 2005.
Rights and permissions
About this article
Cite this article
Pankratov, A.N., Khmelev, S.S. Protolytic properties of cyanic and thiocyanic acids and their isoforms. J Struct Chem 46, 404–408 (2005). https://doi.org/10.1007/s10947-006-0117-y
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10947-006-0117-y