Abstract
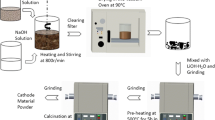
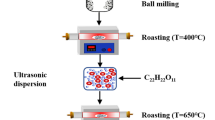
Mechanical activation was shown to be a successful method for preparation of highly dispersed cathode materials (LiMn2O4, LiCoO2, LiV3O8, Li3Fe2(PO4)3, LiTi2(PO4)3) and solid inorganic lithium ion electrolytes (Li1.3Al0.3Ti1.7(PO4)3) for lithium rechargeable batteries. The materials are characterized by sub-micron particle size and a structural disorder (cation vacancies, cation mixing, etc.). This is favorable for electrochemical properties (cycling) of cathode materials, i.e., an increase in both practical capacity and enhanced stability during lithium ion intercalation-deintercalation. However, these advantages are gained only when the cycling begins with intercalation of lithium ions, in other words, with discharge. Li-ion conductivity of Li1.3Al0.3Ti1.7(PO4)3 samples obtained by mechanical activation is two times as large as that of sintered samples due to the absence of insulating impurities.
Similar content being viewed by others
References
B. Scrosati, Electrochim. Acta, 45, 2461–2466 (2000).
A. K. Padhi, K. S. Nanjundaswamy, and J. B. Goodenough, J. Electrochem. Soc., 144, 1188–1194 (1997).
C. W. Kwon, S. J. Hwang, A. Poquet, et al., New Trends in Intercalation Compounds for Energy Storage, Ed. C. Julien et al., Kluwer, Dordrecht (2002), pp. 439–446.
N. Kosova and E. Devyatkina, Ann. Chim. Sci. Mat., 27, 77–90 (2002).
E. Avvakumov, M. Senna, and N. Kosova, Soft Mechanochemical Synthesis: a Basis for New Chemical Technologies, Kluwer, Boston (2001).
P. Barboux, J. M. Tarascon, and F. K. Shokoohi, J. Solid State Chem., 94, 185–196 (1991).
N. V. Kosova, N. F. Uvarov, E. T. Devyatkina, and E. G. Avvakumov, Solid State Ionics, 135, 107–114 (2000).
N. V. Kosova, E. T. Devyatkina, and S. G. Kozlova, J. Power Sources, 97/98, 406–411 (2001).
V. Manev, A. Momchilov, A. Nassalevska, et al., ibid., 54, 501–506 (1995).
N. V. Kosova, S. V. Vosel, V. F. Anufrienko, et al., J. Solid State Chem., 160, 444–449 (2001).
A. S. Andersson, B. Kalska, P. Eyob, et al., Solid State Ionics, 140, 63–70 (2001).
S. Patoux and C. Masquelier, Proc. of the 11 Intern. Meet. on Lithium Batteries, F. McLarnon (ed.), Monterey, California (2002), p. 321.
T. Brousse, P. Fragnaud, R. Marchand, et al., J. Power Sources, 68, 412–415 (1997).
H. Aono, E. Sugimoto, Y. Sadaoka, et al., J. Electrochem. Soc., 136, 590–595 (1989).
Author information
Authors and Affiliations
Additional information
Original Russian Text Copyright © 2004 by N. V. Kosova, E. T. Devyatkina, and D. I. Osintzev
__________
Translated from Zhurnal Strukturnoi Khimii, Vol. 45, Supplement, pp. 144–148, 2004.
Rights and permissions
About this article
Cite this article
Kosova, N.V., Devyatkina, E.T. & Osintzev, D.I. Highly dispersed materials for rechargeable lithium batteries: Mechanochemical approach. J Struct Chem 45 (Suppl 1), S142–S146 (2004). https://doi.org/10.1007/s10947-006-0109-y
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10947-006-0109-y