Abstract
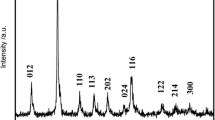
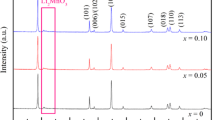
The layered Li-rich Mn-based cathode materials Li[Li0.2Mn0.54Ni0.13Co0.13]O2 were prepared by using co-precipitation technique at different temperatures, and their crystal microstructure and particle morphology were observed and analyzed by XRD and SEM. The electrochemical properties of these samples were investigated by using charge-discharge tests, electrochemical impedance spectroscopy (EIS), and cyclic voltammetry (CV), respectively. The results indicated that all samples are of high purity. When the precursors were co-precipitated at 50 °C, their cathode materials have the most uniform and full particles and exhibit the highest initial discharge capacity (289.4 mAh/g at 0.1C), the best cycle stability (capacity retention rate of 91.2 % after 100 cycles at 0.5C), and the best rate performance. The EIS results show that the lower charge transfer resistance of 50 °C sample is responsible for its superior discharge capacity and rate performance.













Similar content being viewed by others
References
Nguyen MY, Nguyen DH, Yoon YT (2012) A new battery energy storage charging/discharging scheme for wind power producers in real-time markets. Energies 5:5439–5452
Divya KC, Østergaard J (2009) Battery energy storage technology for power systems—An overview. Electr Power Syst Res 79:511–520
Shi SJ, Tu JP, Mai YJ, Zhang YQ, Gu CD, Wang XL (2012) Effect of carbon coating on electrochemical performance of Li1.048 Mn0.381 Ni0.286 Co0.286 O2 cathode material for lithium-ion batteries. Electrochim Acta 63:112–117
Martha SK, Nanda J, Veith GM, Dudney NJ (2012) Electrochemical and rate performance study of highvoltage lithium-rich composition: Li1.2 Mn0.525 Ni0.175 Co0.1 O2. J Power Sources 199:220–226
Croy JR, Gallagher KG, Balasubramanian M, Chen ZH, Ren Y, Kim DH, Kang SH, Dees DW, Thackeray MM (2013) Examining hysteresis in composite xLi2 MnO3 •(1−x)LiMO2 cathode structures. J Phys Chem C 117:6525–6536
Mohanty D, Kalnaus S, Meisner RA, Rhodes KJ, Li JL, Payzant EA, Wood DL III, Daniel C (2013) Structural transformation of a lithium-rich Li1.2 Co0.1 Mn0.55 Ni0.15 O2 cathode during high voltage cycling resolved by in situ Xray diffraction. J Power Sources 229:239–248
Zhao TL, Chen S, Li L, Zhang XF, Chen RJ, Belharouak I, Wu F, Amine K (2013) Synthesis, characterization, and electrochemistry of cathode material Li[Li0.2 Co0.13 Ni0.13 Mn0.54]O2 using organic chelating agents for lithium-ion batteries. J Power Sources 228:206–213
Jin X, Xu QJ, Yuan XL, Zhou LZ, Xia YY (2013) Synthesis, characterization and electrochemical performance of Li[Li0.2 Mn0.54 Ni0.13 Co0.13]O2 cathode materials for lithium-ion batteries. Electrochim Acta 114:605–610
Zheng JM, Wu XB, Yang Y (2011) A comparison of preparation method on the electrochemical performance of cathode material Li[Li0.2 Mn0.54 Ni0.13 Co0.13]O2 for lithium ion battery. Electrochim Acta 56:3071–3078
Wang CL, Zhou F, Ren C, Wang YF, Kong JZ, Jiang YX, Yan GZ, Li JX (2015) Influences of carbonate coprecipitation temperature and stirring time on the microstructure and electrochemical properties of Li1.2 [Mn0.52 Ni0.2 Co0.08]O2 positive electrode for lithium ion battery. Solid State Ionics 281:96–104
Ma SM, Hou XH, Lin ZR, Huang YL, Gao YM, Hu SJ, Shen JD (2016) One-pot facile co-precipitation synthesis of the layered Li1+x (Mn0.6 Ni0.2 Co0.2)1−x O2 as cathode materials with outstanding performance for lithium-ion batteries. J Solid State Electrochem 20:95–103
Kong JZ, Ren C, Jiang YX, Zhou F, Yu C, Tang WP, Li H, Ye SY, Li JX (2016) Li-ion-conductive Li2TiO3 -coated Li[Li0.2 Mn0.51 Ni0.19 Co0.1]O2 for high-performance cathode material in lithium-ion battery. J Solid State Electrochem 20:1435–1443
Zhu ZY, Zhu LW (2014) Synthesis of layered cathode material 0.5Li2MnO3•0.5LiMn1/3Ni1/3Co1/3O2by an improved co-precipitation method for lithium-ion battery. J Power Sources 256:178–182
Shi SJ, Tu JP, Tang YY, Yu YX, Zhang YQ, Wang XL (2013) Synthesis and electrochemical performance of Li1.131Mn0.504Ni0.243Co0.122O2 cathode materials for lithium ion batteries via freeze drying. J Power Sources 221:300–307
Shi SJ, Tu JP, Tang YY, Yu YX, Zhang YQ, Wang XL, Gu CD (2013) Combustion synthesis and electrochemical performance of Li[Li0.2Mn0.54Ni0.13Co0.13]O2with improved rate capability. J Power Sources 228:14–23
Kim GY, Yi SB, Park YJ, Kim HG (2008) Electrochemical behaviors of Li[Li(1- x)/3Mn(2- x)/3Nix/3Cox/3]O2cathode series (0<x<1) synthesized by sucrose combustion process for high capacity lithium ion batteries. Mater Res Bull 43:3543–3552
Wang CL, Zhou F, Chen KM, Kong JZ, Jiang YX, Yan GZ, Li JX, Yue C, Tang WP (2015) Electrochemical properties of a-MoO3-coated Li[Li0.2Mn0.54Ni0.13Co0.13]O2cathode material for Li-ion batteries. Electrochim Acta 176:1171–1181
Wang CL, Zhou F, Ren C, Kong JZ, Tang Z, Li JX, Yu C, Tang WP (2015) Influence of Co increment on the electrochemical properties of Li1.2[Mn0.52−0.5xNi0.2−0.5xCo0.08+x]O2cathode materials for lithium-ion batteries. J Alloys Compd 643:223–230
Jin X, Xu QJ, Liu HM, Yuan XL, Xia YY (2014) Excellent rate capability of Mg doped Li[Li0.2Ni0.13Co0.13Mn0.54]O2cathode material for lithium-ion battery. Electrochim Acta 136:19–26
Zheng JM, Wu XB, Yang Y (2013) Improved electrochemical performance of Li[Li0.2Mn0.54Ni0.13Co0.13]O2cathode material by fluorine incorporation. Electrochim Acta 105:200–208
Qing RP, Shi JL, Xiao DD, Zhang XD, Yin YX, Zhai YB, Gu L, Guo YG (2016) Enhancing the kinetics of Li-Rich cathode materials through the pinning effects of gradient surface Na+Doping. Adv Energy Mater 6:1501914
Shi SJ, Tu JP, Tang YY, Liu XY, Zhang YQ, Wang XL, Gu CD (2013) Enhanced cycling stability of Li[Li0.2Mn0.54Ni0.13Co0.13]O2by surface modification of MgO with melting impregnation method. Electrochim Acta 88:671–679
Lu C, Wu H, Zhang Y, Liu H, Chen BJ, Wu NT, Wang S (2014) Cerium fluoride coated layered oxide Li1.2Mn0.54Ni0.13Co0.13O2 as cathode materials with improved electrochemical performance for lithium ion batteries. J Power Sources 267:682–691
Liu XY, Liu JL, Huang T, Yu AS (2013) CaF2-coated Li1.2Mn0.54Ni0.13Co0.13O2 as cathode materials for Liion batteries. Electrochim Acta 109:52–58
Kong JZ, Wang CL, Qian X, Tai GA, Li AD, Wu D, Li H, Zhou F, Yu C, Sun Y, Jia D, Tang WP (2015) Enhanced electrochemical performance of Li1.2Mn0.54Ni0.13Co0.13O2 by surface modification with graphene-like lithium-active MoS2. Electrochim Acta 174:542–550
Cong LN, Gao XG, Ma SC, Guo X, Zeng YP, Tai LH, Wang RS, Xie HM, Sun LQ (2014) Enhancement of electrochemical performance of Li[Li0.2Mn0.54Ni0.13Co0.13]O2by surface modification with Li4Ti5O12. Electrochim Acta 115:399–406
Wang ZY, Liu EZ, He CN, Shi CS, Li JJ, Zhao NQ (2013) Effect of amorphous FePO4 coating on structure and electrochemical performance of Li1.2Ni0.13Co0.13Mn0.54O2 as cathode material for Li-ion batteries. J Power Sources 236:25–32
Zheng FH, Yang CH, Xiong XH, Xiong JW, Hu RZ, Chen Y, Liu ML (2015) Nanoscale surface modification of lithium-rich layered-oxide composite cathodes for suppressing voltage fade. Angew Chem Int Ed 54:13058–13062
Yan PF, Xiao L, Zheng JM, Zhou YG, He Y, Zu XT, Mao SX, Xiao J, Gao F, Zhang JG, Wang CM (2015) Probing the degradation mechanism of Li2MnO3Cathode for Li-ion batteries. Chem Mater 27:975–982
Yabuuchi N, Yoshii K, Myung ST, Nakai I, Komaba S (2011) Detailed studies of a high-capacity electrode material for rechargeable batteries, Li2MnO3-LiCo1/3Ni1/3Mn1/3O2. J Am Chem Soc 133:4404–4419
Thackeray MM, Kang SH, Johnson CS, Vaughey JT, Benedek R, Hackney SA (2007) Li2MnO3-stabilized LiMO2(M = Mn, Ni, Co) electrodes for lithium-ion batteries. J Mater Chem 17:3112–3125
Yoon WS, Iannopollo S, Grey CP, Carlier D, Gorman J, Reed J, Ceder G (2004) Local structure and cation ordering in O3 lithium nickel manganese oxides with stoichiometry LiNiXMn(2-x)/3Li(1-2x)/3O2. Electrochem Solid State Lett 7(7):A167–A171
Li J, Klopsch R, Stan MC, Nowak S (2011) Synthesis and electrochemical performance of the high voltage cathode material Li[Li0.2Mn0.56Ni0.16Co0.08]O2with improved rate capability. J Power Sources 196:4821–4825
Cho SW, Kim GO, Ryu KS (2012) Sulfur anion doping and surface modification with LiNiPO of a Li[Co0.1Ni0.15Li0.2Mn0.55]O2 cathode material for Li-ion batteries. Solid State Ionics 206:84–90
Nobili F, Croce F, Tossici R, Meschini I (2012) Sol–gel synthesis and electrochemical characterization of Mg-/Zr-doped LiCoO2cathodes for Li-ion batteries. J Power Sources 197:276–284
Kang SH, Thackeray MM (2009) Enhancing the rate capability of high capacity xLi2MnO3•(1-x)LiMO2(M =Mn, Ni, Co) electrodes by Li–Ni–PO4 treatment. Electrochem Commun 11:748–751
Yi TF, Tao W, Chen B, Zhu YR, Yang SY, Xie Y (2016) High-performance xLi2MnO3• (1-x) LiMn1/3Co1/3Ni1/3O2(0.1<x<0.5) as cathode material for lithium-ion battery. Electrochim Acta 188:686–695
Robertson AD, Bruce PG (2002) The origin of electrochemical activity in Li2MnO3. Chem Commun 2790–2791
Thackeray MM, Johnson CS, Vaughey JT, Li N, Hackney SA (2005) Advances in manganese-oxide ‘composite’ electrodes for lithium-ion batteries. J Mater Chem 15:2257–2267
Yabuuchi N, Kubota K, Aoki Y, Komaba S (2016) Understanding particle-size-dependent electrochemical properties of Li2MnO3-Based positive electrode materials for rechargeable lithium batteries. J Phys Chem C 120:875–885
Croy JR, Kim D, Balasubramanian M, Gallagher K, Kang SH, Thackeray MM (2012) Countering the voltage decay in high capacity xLi2MnO3•(1–x)LiMO2Electrodes (M=Mn, Ni, Co) for Li+−Ion batteries. J Electrochem Soc 159:A781–A790
Ito A, Li DC, Sato Y, Arao M, Watanabe M, Hatano M, Horie H, Ohsawa Y (2010) Cyclic deterioration and its improvement for Li-rich layered cathode material Li[Ni0.17Li0.2Co0.07Mn0.56]O2. J Power Sources 195:567–573
Lu ZH, Dahn JR (2002) Understanding the anomalous capacity of Li/Li[NixLi(1/3−2x/3)Mn(2/3−x/3)]O2Cells using in situ x-ray diffraction and electrochemical studies. J Electrochem Soc 149:A815–A822
Karthikeyana K, Amaresha S, Lee GW, Aravindana V, Kim H, Kang KS, Kim WS, Lee YS (2012) Electrochemical performance of cobalt free, Li1.2 (Mn0.32Ni0.32Fe0.16)O2cathodes for lithium batteries. Electrochim Acta 68:246–253
Zhang JC, Zhang H, Gao R, Li ZY, Hu ZB, Liu XF (2016) New insights into the modification mechanism of Li-rich Li1.2Mn0.6Ni0.2O2 coated by Li2ZrO3. Phys Chem Chem Phys 18:13322–13331
Li LJ, Chen ZY, Zhang QB, Xu M, Zhou X, Zhu HL, Zhang KL (2015) A hydrolysis-hydrothermal route for the synthesis of ultrathin LiAlO2-inlaid LiNi0.5Co0.2Mn0.3O2 as a high-performance cathode material for lithium ion batteries. Mater Chem A 3:894–904
Johnson CS, Li NC, Lefief C, Vaughey JT, Thackeray MM (2008) Synthesis, characterization and electrochemistry of lithium battery electrodes: xLi2MnO3•(1 - x)LiMn0.333Ni0.333Co0.333O2(0<=x<=0.7). Chem Mater 20:6095–6106
Acknowledgments
This work was financially supported by the Special Fund of the Scientific and Technological Achievements Transformation Project in Jiangsu Province (No. BA2013142), Jiangsu Province Natural Funds for the Central Universities (No. BK20130800), Fundamental Research Funds for the Central Universities (No. NS2014054), Funding of Shanghai Academy of Spaceflight Technology (No. SAST201371), and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). We would like to acknowledge them for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• The Li-rich layered Li[Li0.2Mn0.54Ni0.13Co0.13]O2 powders were synthesized via using a co-precipitation method and a two-step solid-state reaction process.
• All Li[Li0.2Mn0.54Ni0.13Co0.13]O2 samples demonstrate hexagonal α-NaFeO2 structure, and the size of spherical particles varies between 100 and 400 nm.
• The 50 °C sample shows the highest initial discharge capacity (289.4 mAh/g) and the highest initial Coulombic efficiency (81.5 %).
• The 50 °C sample delivers highest discharge capacity at each current density and shows the best rate performance.
Rights and permissions
About this article
Cite this article
Jiang, Y., Zhou, F., Wang, C. et al. Influence of co-precipitation temperature on microstructure and electrochemical properties of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode materials for lithium ion batteries. Ionics 23, 585–596 (2017). https://doi.org/10.1007/s11581-016-1863-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-016-1863-2