Abstract
Mono and bimetallic modified MCM-41(Mobil Composition of Matter No. 41): Zn-MCM (ZM), Zn/Co-MCM41 (ZCM), and Zn/Pd-MCM-41 (ZPM) molecular sieves were produced by a surfactant-assisted technique. The structural and textural features were examined through spectroscopic and analytical techniques. The XRD analysis indicated broadening of diffraction peaks and a shift towards higher 2-theta values in the metal-incorporated (M-MCM-41) samples, confirming the successful integration of metal atoms into the MCM-41 framework; it also highlighted the preservation of a hexagonal structure with reasonable regularity, emphasizing the influence of metal incorporation on the mesoporous architecture of MCM-41. N2 adsorption–desorption isotherms revealed type IV isotherms for all samples; the BET specific surface area decreased to 672.48, 667.90, and 562.50 m2/g in ZM, ZCM, and ZPM, respectively comparing to the unincorporated MCM-41 sample (1200 m2/g), indicating partial filling of mesopores by metal centers, as confirmed by TEM images. The diffuse reflectance spectra exhibited a noteworthy optical band gap reduction of MCM-41 (5.98 eV) upon the incorporation of Zn and Co/Zn ions, resulting in values of 5.86 and 5.24 eV, respectively, with refractive index values close to 2. Additional absorption bands energies are observed at 3.14, 3.18, and 1.70 eV in ZM, ZPM, and ZCM samples, respectively suggesting the suitability of the metal incorporated samples for the photocatalytic applications. The M-incorporated samples exhibited a decline in the transmission intensity accompanied by small shifts. The enhanced antimicrobial activity of the metal-incorporated samples, surpassing that of the pure MCM-41 against a variety of tested microorganisms, is attributed to the presence of incorporated metal species, which create a more acidic environment and substantially contribute to the heightened antimicrobial effectiveness. The ZM compound demonstrated potent inhibition against Bacillus cereus and Pseudomonas aeruginosa bacteria, displaying comparable efficacy to Ampicillin, as a reference antibiotic. Additionally, ZPM exhibited considerable inhibitory activity against Escherichia coli, surpassing the reference antibiotic and showing similar effectiveness against Bacillus cereus, Pseudomonas aeruginosa, and Salmonella typhimurium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The impressive structural features of well-organized mesoporous molecular sieve materials like MCM-41, including high surface area, substantial pore-volume, uniform pore networks, versatile surface functionalities, and robust physicochemical properties, emphasize their considerable potential in a wide range of applications. These applications cut across diverse technological domains, including but not limited to catalysis, gas storage, adsorption/separation, solar cells, sensors, natural rubber fillers, chromatography, light-harvesting. and optoelectronics, [1,2,3,4], and additional pivotal domains of discipline. Significantly, mesoporous materials demonstrate biocompatibility and non-toxicity, making them exceptionally well-suited for utilization in biotechnology applications. This outstanding attribute positions them as biomaterials playing crucial roles in sensing, drug delivery, and biochemical technologies [5, 6]. Nevertheless, the raw mesoporous silica, specifically MCM-41, exhibits restricted inherent chemical reactivity attributable to the distinctive silica structure, resulting in a scarcity of active sites and thereby constraining its practical applications. Numerous studies have addressed this limitation through modifications targeting the active sites of MCM-41 mesoporous silica, employing methods such as organosilane functionalization [7, 8], polymer modification [9, 10], and the incorporation or impregnation of metals [11,12,13,14].
The introduction of heteroatoms mesoporous silica significantly impacts the properties and reactivity of the resulting materials [15, 16]. By employing this approach, not only are there improvements observed in the physicochemical features, but it also leads in enhancement in the ion-exchange property and the creation of a significant quantity of active sites [17,18,19]. The most crucial contenders for MCM-41 dopants include the third main group as well as certain trivalent transition metal. [2, 20,21,22,23]. Furthermore, the incorporation of M2+ and M4+ into the structure of mesoporous silica has been investigated. This substitution led to a slight distortion of the tetrahedral structure and further oxidizing the dopant ions specifically (M2+ → M3+ and M4+ → M5+) [24]. It has been reported that highly reactive metals could be attached onto both the external surface and inner pores [25, 26].
From a biological aspect, inclusion of metal ions into the silica framework introduces unique functionalities with potential applications in improved biological activities. Additionally, the modification of mesoporous silica through metal ion doping influences its surface chemistry, allowing the introduced metal ions to serve as active centers for binding biomolecules. This interaction is particularly significant in biomedical application including antimicrobial [27] and drug delivery systems [28]. The modification induces changes in the mesoporous silica's framework, leading to alterations in its physicochemical properties. Notably, the introduction of metal ions brings about several changes in the material's framework, including the formation of acidic sites [29, 30]. This alteration enhances catalytic activity, providing a conducive environment for various biological processes. The presence of metal-doped sites catalyzes specific reactions, making them pivotal for biological applications such as antibacterial or antifungal activities [27, 31].
The broad utilization of antibiotics in current times has resulted in the escalation of drug resistance among bacteria, leading to the appearance new type of antibiotic-resistant microorganism known as super bacteria [32]. The increasing prevalence of these microorganism is a major global concern for well-being of all living organisms. As a result, it is now more important than ever to prioritize the discovery and development of antimicrobial agents that possess strong efficacy against various types of pathogens and are not prone to resistance [33,34,35]. MCM-41 was hydrothermally prepared by Boubekeur Asli et al., and subsequently underwent modification with silver species. The samples obtained underwent testing for methylene blue (MB) reduction in the existence of NaBH4. Additionally, they were evaluated for their antibacterial properties against various bacteria. The Ag-MCM-41 and AgNPs-MCM-41 exhibited superior efficacy in inhibiting pathogenic bacteria [36]. Another study by Copcia et al. [37] revealed that the antimicrobial effectiveness of SBA-15 loaded with ZnO NPs was superior to that of clinoptilolite loaded with nano zinc oxide. Donnadio et al. [38] developed microbicidal and fungicidal nano zinc oxide compositions supported on silica dioxide. Anna Donnadio reported ZnO supported on mesoporous silicas demonstrating potential activity against fungi and bacteria [38, 39]. Concurrently, in the present investigation, a pure highly ordered MCM-41, along with Zn-monoincorprated-MCM-41 and bimetallic-incorporated (Zn/Co)-; (Zn-Pd)-MCM-41, were prepared using a surfactant-aided precipitation process. The impact of metal ions (Zn, Co and Pd) incorporation on the structure, morphology of MCM-41 framework was explored. The textural and optical features was also reported. This approach is anticipated to modify the physicochemical properties of MCM-41 by creating various active sites, leading to improved optical attributes and increased ion-exchange capacity. The antimicrobial efficacy of the prepared samples against both Gram- positive and Gram-negative bacteria, as well as fungi was evaluated. Combination of ZnO with (Co/Pd) and MCM-41 mesoporous material is anticipated to have a synergistic effect on microbial activity.
2 Materials and experimental methods
Hexa decyl trimethyl-ammonium bromide (CTAB), MW = 364.45, Acros Organic, Belgium. Sodium silicate (Na2SiO3), MW = 122.06. Zinc nitrate hexahydrate extra pure (Zn(NO3)2·6H2O), MW = 297.48, Alphachmemika, India. Cobaltous nitrate hexahydrate purified LR (Co(NO3)2·6H2O), MW = 291.03, Palladium nitrate (Pd(NO3)2), MW = 230.43, Ld.Fine-Chemlid. Sodium hydroxide pellets, MW = 40 g/mol, SIGMA-ALDRICH.
2.1 Preparation of pure MCM-41 support by precipitation method
MCM-41 was prepared using the following procedures: typically, dissolving of 3.64 gm of CTAB (e.g., cetyltrimethylammonium bromide) in 89.9 mL dist.H2O was followed by maintained stirring (at a stirring rate of 500 rpm) at 55 °C for 1 h to obtain homogeneous solution (solution I). A second solution (solution II) was obtained by dissolving 10.158 gm of Na2SiO3 in an aqueous solution of NaOH (16 mM). Then the dropwise addition of solution (II) to solution (I) followed by adjusting the pH of the mixture at 10 using dropwise addition of NaOH and H2SO4. the resulting mixture was subjected to continuous stirring at room temperature for a duration of 24 h. The collected precipitate was thoroughly rinsed with distilled water and ethanol multiple times. Subsequently, the sample was dried at a temperature of 70 °C and then subjected to calcination at 550 °C for a period of 6 h. Whereas, pure MCM-41 is coded as pure-M.
2.2 Preparation of mono Zn and bimetallic Zn-Co and Zn-Pd-MCM-41
Zn-, Zn-Co- and Zn-Pd- modified MCM-41 were prepared in aqueous (water) media using Zn(NO3)2·6H2O, Co(NO3)2·6H2O and Pd(NO3)2 as the metal ions precursors. In a typical preparation, 3.0 g of uncalcined CTAB-MCM-41 was disseminated in 40 mL H2O. Then a solution containing the desired amount of metal precursor source according to the M/Si atomic percentage (8%Zn; 4%Zn4%Co and 4%Zn4%Pd) was poured in the CTAB-MCM-41 solution. The mixtures were allowed to stir at room temperature for 1 h. after that the solutions were stirred (stirring rate of 500 rpm) at 80 °C for 5 h. The obtained powders were then air-dried at 70 °C for 24 h. The elimination of organic materials from the mesopores was achieved through the process of calcination, which involved subjecting the sample to air at a temperature of 550 °C for a period of 6 h. For clarity, the Zn-MCM-41, Zn-Co-MCM-41 and Zn-Pd-MCM-41 thus obtained are coded as ZM, ZCM and ZPM, respectively.
2.3 Characterization techniques
The characterization of the materials was performed using X-ray diffraction (XRD) on (XRD, PANalytical X-ray diffraction equipment model X′Pert PRO). N2 adsorption/desorption isotherm was analyzed at − 196 K °C using NOVA 3200 equipment, USA. The samples were initially out-gassed under vacuum (10–4 Torr) at 300 °C. BET surface areas (SBET) were calculated from the adsorption branch of the isotherm by the aid of the BET equation. Pore size distributions (PSD) were estimated using (DFT) method from the adsorption branch of the isotherm.; The particles morphology and size were analyzed using a scanning electron microscope with energy dispersive spectroscopy (SEM/EDS, model Quanta 250 FEG) and transmission electron microscope (TEM, JEOL JEM-2100); whereas the transmittance spectra in the infrared region were obtained with FTIR-ATR Brucker Vertex 80 V with resolution 4 cm−1 in the range of 4000–400 cm−1; Diffuse reflectance UV–vis spectroscopic measurements were carried out on a double beam spectrophotometer-JASCO (model V-570 UV–Vis-NIR). The absorption intensity was calculated from the Schuster-Kubelka–Munk equation function \(\frac{(1 - R_d )}{{2R_d }}^2 = \frac{ \propto }{S}\) where, FR is the Kubelka–Munk function corresponding to the absorbance, Rd is the diffuse reflectance, α is the absorption coefficient and in the scattering coefficient. The bandgap values were determined using the equation \(( \propto h\nu )^{{\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 \gamma }}\right.\kern-0pt}\!\lower0.7ex\hbox{$\gamma $}}} = A(h\nu - Eg)\) where α is the absorption coefficient, ν is the light frequency, A is the proportionality constant, and Eg is the bandgap energy, γ is 0.5 and 2 for direct and indirect transitions, respectively.
2.4 Biological activity
The antibacterial activities were carried out using the diffusion plate method. Tested samples (1 mg per each treatment) were fixed on nutrient agar spread on a 9-cm diameter plate. The nutrient agar was seeded with a suspension of spores from the test organism. The sample was incubated at a temperature of 37 °C for 24 h in order to allow the bacteria to grow. Following the incubation period, the diameter of the zone of inhibition around the sample was measured. The sample's effectiveness in inhibiting the growth of the specific test organism was evaluated according to diameter of the inhibition clear zone. The microbicidal activity of samples was inspected with Gram positive bacteria, Bacillus cereus, staphylococcus aureus ATCC 6538, and Gram-negative bacteria Escherichia coli NRRN 3008, pseudomonas aeruginosa ATCC 10145, Salmonella typhimurium ATCC 25566 and pathogen yeast candida albicans EMCC 105. The obtained results are compared with the Ampicillin as a reference antibiotic.
3 Results and discussion
3.1 XRD analysis
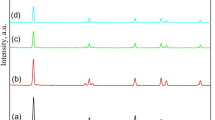
Figure 1 exhibit the X-ray diffraction covering the range of 2°–10° 2θ for the pure MCM-41 (pure-M) support and the Zn, Zn/Co, and Zn/Pd-MCM-41 samples. The XRD pattern of pure-M reveals four distinct peaks, characteristic of a well-defined MCM-41. Specifically, the peaks at 2θ 2.33°, 4.02°, and 4.61° correspond to the (100), (110), and (200) planes of a hexagonal symmetry, indicative of regular mesopores [40]. Furthermore, the peak at 6.12° associated with the 300 Miller index, is occasionally observed in exceptionally well-ordered MCM-41 structures.
Upon closer examination (Fig. 1b) of the XRD spectra, some variations become apparent when comparing the patterns of pure MCM-41 and the M-MCM-41 samples. Generally, a noticeable broadening is detected in the main diffraction peak (100) of pure-M. Additionally, there is a discernible shift to higher 2θ degrees. Notably, the other three peaks (110), (200), and (300) are no longer present. The intensity of the (100) peak is observed to be less in ZPM than in ZCM, and in turn, less in ZCM than in ZM sample. This intensity attenuation in M-MCM-41 samples compared to the pure sample suggests a broader pore diameters-distribution of and a decline in the mesopores ordering. The peaks-broadening, disappearance, and intensity-reduction collectively serve as typical indicators of the insertion of metal into the silica framework [41, 42]. These structural changes in the XRD patterns feature the influence of metal incorporation on the MCM-41 architecture, providing valuable insights into the modification of its crystalline structure. The introduction of metal contributes to a further disordering of the metal ions modified MCM-41 channels; nevertheless, the hexagonal ordering is still preserved with rational regularity. An intriguing observation worth noting is the shift of the (100) diffraction peak to higher 2θ degrees (in comparison to pure MCM-41), signifying an enhancement in the metal species-MCM-41 interaction [43]. However, this shift is more pronounced in ZM and ZCM (0.125° and 0.105° respectively) than ZPM (0.045°). This interestingly suggests a unit cell shrinkage, leading to a decrease in the distance of pore–pore centers.
To provide a comparative estimation of pore size diameters of the pure and M-MCM-41 samples, the value of the cell parameter a0 was estimated. The center-to-center distance between the two pores (a0) was calculated using the equation: \(a_0 = 2d_{100} /\sqrt {3}\). The d-spacing for (100) peak of pure MCM-41 is 37.95 Å, matching a0 = 43.8 Å. Powder X-ray diffraction of ZM, ZCM, and ZPM samples, respectively, revealed a decrease in the d100 spacing to 36.02, 36.31, and 37.23 Å, corresponding to pore repeat distances a0 of 41.6, 41.9, and 43.0 Å, respectively. The a0 of M-MCM-41 exhibits a slight reduction compared to the pristine sample. This decrease in the a0 is likely attributed to a contraction in the framework caused by Si-substitution by M. It is supposed that the lack of strict atomic-level crystallographic ordering in MCM-41 and the varying polymerization ability of the material contribute to the variations in the cell parameters. The relatively low degree of polymerization, due to the facile condensation of M-silicate polyanions, is insufficient to elevate a0 value. The primary mesopore diameter (Wd) was computed utilizing a0 values, employing the equation \(W_d = cd\sqrt {(\rho V_p /(1 + } \rho V_p )\) (c: constant = 1.213, d: (100) plane-spacing, ρ: pore wall-density, and Vp: primary mesopore volume) [14, 44, 45]. A comparison of Wd of pure-M and M- MCM-41 compositions reveals a small decline (0.07 nm) in ZPM and a pronounced decline (0.19 and 0.16 nm) in ZM and ZCM samples, respectively. These results demonstrated successful modification of MCM-41 structure upon metal-incorporation and tuning of unit cell parameter, pore diameter, of MCM-41 which provides valuable insights for tailoring its properties for diverse applications in materials science and catalysis.
3.2 BET analysis
Figure 2a illustrates the complete N2 adsorption–desorption isotherms for all samples across a range of P/P0 of(0.005 to 1.00). The isotherm behavior of each synthesized material can be characterized as type IV [46]. In the low-pressure section (P/P0 < 0.1), all samples display an inclined adsorption isotherm, suggesting the presence of micropores and a robust interaction between the materials and nitrogen. The strong adsorption potential of micropores results in a single molecular layer on the pore-surface. A characteristic hysteresis loop was observed for the pure MCM-41 support in the range of 0.30–0.60 (Fig. 2a) ascribed to capillary condensation within the mesopores. The steep slope indicates a high level of uniformity in the size of the pores, which is in agreement with the findings from XRD analysis. Between relative pressures of 0.6 and 0.8, the MCM-41 isotherm exhibits a relatively stable adsorption region. At high pressures (0.8 < P/P0 < 1.0), the isotherms rise rapidly, and near a relative pressure of 1, no plateau is observed, indicating the absence of an adsorption saturation phenomenon. This suggests that gas is primarily adsorbed on larger pores (mesopores or macropores), leading to a significant increase in gas adsorption capacity. The calcined pure-M sample displayed a BETsurface area of 1200 m2/g.
Significant differences observed in the multilayer adsorption zone were observed when comparing the isotherms of M-MCM-41 samples with the parent pure-Msample. These differences stem from unlike surface features between pure and incorporated samples. M-MCM-41 samples exhibit a lower maximum adsorbed volume, consistent with the observed reduced BET surface area. The slope associated with the capillary condensation, in the P/P0 ranges of (0.43–0.93), (0.43–0.95), and (0.38–0.94), is less pronounced for M-MCM-41 samples than for the pure material. This suggests a broader distribution of pore diameters in M-MCM-41 samples. At higher relative pressures, there is an increase in adsorption in M-MCM-41 samples due to multilayers adsorption on the mesoprous exterior surface. The type of hysteresis loop observed is indicative of the pore network's influence. Furthermore, the M-MCM-41 samples display an H3 hysteresis loop, this suggests the presence of narrow, slit-like mesopores in the materials [47, 48].
Table 1 presents an overview of the textural properties of the synthesized materials determined from adsorption–desorption isotherms analysis. Pure-Msample exhibited BETsurface area of 1200 m2/g, that was reduced to 672.48, 667.90, and 562.50 m2/g in the ZM, ZCM, and ZPM samples, respectively. The decreasing in the BETsurface area after metal loading could be accredited to the filling of mesopores and the obstruction of pore entrances of MCM-41 by the metal centers deposited on the surface. This conclusion is supported by TEM images [25].
The incorporation of metal components has varying impacts on pore blockage. Among the different metals studied, zinc (Zn) resulted in a comparatively lower reduction in surface area. In contrast, the combination of zinc and cobalt (Zn–Co) exhibited a greater degree of pore blockage. However, the most substantial decline in surface area was observed using zinc and palladium (ZPM) coupling [49]. This could be due to the migration of certain palladium species within the MCM-41 framework, leading to the blocking of mesopores [47, 48].
Figure 2b displays the DFT pore size distribution for unmodified as well as M-modified MCM-41 samples. The pure-M revealed a tightly packed distribution of mesopores, while the M-MCM-41 samples exhibited noticeable variation in their pore size distributions. The less uniform distribution of pore sizes in the M-MCM-41 samples specifies existence of some disorder, potentially stemming from irregularly arranged pores and/or a higher defects concentration in the pore walls.
The ZM and ZCM samples recorded smaller average pore diameters (Wd) (2.65, 2.65 nm) compared to pure-M. however, the ZPM showed a higher Wd of 3.63 nm aligning with the XRD findings. The mesoporous volume (Vp) follows a similar pattern to Dp; decreased in ZM and ZCM samples while markedly increased in the ZPM sample. The variation in pore size distribution could be attributed to its amorphous structure of MCM-41, where both bond lengths and angles are subject to change. The increase of Vp and Dp in the ZPM sample could be attributed to some preferences for Pd ions in increasing the micelle size due to the presence of the charged ions of palladium salt [50].
The estimation of wall thickness was calculated using the equation: Wt = (2/√3) * d100– Dp(DFT). In the case of pure MCM-41, the wall thickness was found to be 0.9 nm. However, for the modified samples ZM, ZCM, and ZPM, the wall thickness values were measured to be 1.51, 1.54, and 0.67 nm, respectively. The results obtained from XRD analysis align with these findings. The rise in wall thickness in the ZM, ZCM samples when compared with pure MCM-41 suggests a modification of the structure due to the introduction of these elements in the framework. Similar findings were reported upon the incorporation of titanium (Ti), vanadium (V), and molybdenum (Mo) into MCM-41 resulted in an increase in the wall thickness. This phenomenon indicates that the introduction of these particular metal elements has an impact on the structural characteristics of the MCM-41 matrix [51,52,53]. Such alterations in wall thickness are significant as they suggest a modification in the internal framework of the material as it likely influences the arrangement of the silica particles within the matrix, leading to a denser or thicker structure. The thicker wall of ZM and ZCM samples could be attributed to an increase in the extent of zinc silicate and cobalt silicate condensation; this enhancement in condensation could account for thicker wall and improved structural stability.
3.3 Scanning electron microscope and EDX
Figure 3 shows SEM images of pure-M, ZM, ZCM and ZPM samples. The scanning electron microscopy analysis of pure-M Fig. 3a. unveils a distinctive morphology characterized by near-spherical structures, encompassing both small (0.1–0.2 µm) and large (4–6 µm) particles. The incorporation of different metal ions, namely Zn, Co, and Pd, into the MCM-41 framework induces diverse and pronounced effects on the material's morphological and structural characteristics. In the case of Zn-MCM-41 (Fig. 3b), the altered morphology, with reduced presence of smaller spheres compared to pure MCM-41, along with the emergence of sheet-like structures and a sponge pattern, signifies a complex interplay between zinc and the MCM-41 matrix. This suggests a unique influence on growth patterns, potentially leading to the formation of a porous structure. Moving to Co-MCM-41, Fig. 3c, the substantial prevalence of sheet-like structures and sacks of tiny particles points to a significant impact of cobalt on crystal growth or aggregation processes. Notably, the absence of large spheres indicates a specific interaction that prevents their formation, demonstrating the selective role of cobalt in shaping the MCM-41 structure. For ZPM, Fig. 3d, the SEM images reveal an aggregate of small well-shaped spheres (40–95 nm), indicating a distinctive particle arrangement resulting from the incorporation of palladium. This further emphasizes the facility of Pd to make specific morphological changes in MCM-41 framework. These observations highlight the versatility of MCM-41 as a host matrix for metal ion incorporation, showing the diverse morphologies and structural modifications achievable by tailoring the composition. The EDX analysis revealed the existence of Silicon (Si) and oxygen (O) as fundamental components, along with Zinc (Zn), cobalt (Co), and palladium (Pd) identified as dopant elements (Fig. 4). Notably, no other elements were detected, providing confirmation of the purity of the synthesized compositions.
3.4 TEM analysis
The TEM images of pure as well as metal (Zn, Zn/Co and Zn/Pd) incorporated MCM-41 compositions and SAED patterns of ZM and ZPM samplesare depicted in Fig. 5, providing crucial insights into the structural characteristics. Figure 5a illustrates the morphology of the pure-M sample, revealing distinct silica microspheres adorned with interconnected, regularly spaced pores. Meanwhile, Fig. 5b shows vertically aligned, uniform lines within the pore channels, signifying the highly ordered nature of the one-dimensional channels in MCM-41.
The large single-phase domain, good mesopore regularity, and ultrathin specimen are indicative of a well-structured MCM-41 sample that are suitable for determining the 3D mesostructure and its space group. The spacing between the centers of the pores (light spots), which corresponds to 'ao,' was measured to be 4.81 ± 0.03 nm, in an agreement with the XRD value (Table 1). The TEM image provided an estimate of the Wd size, which was found to be 3.91 ± 0.04 nm. This measurement falls within the expected range for MCM-41. Examining TEM images of M-MCM-41 (Fig. 5c–h) revealed a similarity in pore characteristics to the sample, albeit there were some irregularities in the morphological and pore ordering, likely resulting from the loading of metal ions. Co, Zn, and Pd incorporated ions, being denser than Si in SiO2 constituting MCM-41, appear as relatively dark spots in TEM images. Several reports referred to such observation for metal-incorporated MCM-41 materials [25, 54]. The average particle sizes of Zn, Zn/Co, and Pd centers were assessed to be 1.47 nm, 2.25 nm, and 5.4 nm, respectively (Fig. 5). These particle sizes, observed as relatively dark spots in the TEM images of ZM and ZCM are smaller than the respective pore diameters (3.57 and 3.60 nm respectively), suggesting possible incorporation within the pores. However, the semi-transparency of MCM-41 to the TEM beam hinders determining whether these incorporated centers are on the surfaces or within the pores. Notably, the visibility of Pd centers in the TEM image does not strongly suggest their location inside the MCM-41-pores, as the Pd-centers dimensions appear relatively larger in comparison to the calculated wd. However, it remains plausible to suggest that some Pd centers are small enough to reside within the pores Fig. 5. Notably, well-defined alternating light and dark parallel lines observed in all samples except ZPM signify the MCM-41 particles boundary pores [44, 55]. The average cell parameters calculated from the distances between middles of adjacent, lighter parallel lines were 4.48 nm and 4.53 nm for ZM and ZCM samples, respectively (Fig. 6). These values are 1.50% and 2.20% higher than their counterpart XRD-derived average cell parameter values (Table 1).
Additionally, the TEM analysis provided an estimation of the Wd, showing a decrease of 2.84% and 2.80% compared to the Wd of the pure-M. These observations correlate with the differences in Wd revealed by XRD analysis between the original material and the MCM-41 samples with incorporated metals.
3.5 Optical properties and band gap
To examine the influence of Zn2+, Zn2+/Co2+, and Zn2+/Pd2+ doping on the optical properties MCM-41, the diffuse reflectance (DR) of Zn2+, Zn2+/Co2+, and Zn2+/Pd2+ doped MCM-41 samples was measure 200–1000 nm range Fig. 7. Significant changes in the absorption property of the pure-M occur following the incorporation of M-doping. The spectrum of pure-M exhibits high reflectance with a slight absorption at 280 nm. This related to the insensitive character of silica, which lacks π and n electrons, making it unresponsive to both UV and visible light. The slight absorption band shifts to lower wavelengths with the incorporation of metal ions. This shift suggests the formation of a new bonding arrangement involving –O–M–O–Si–O– in the metal-doped samples. Thus, it can be concluded that the metal ions are coordinated in a tetrahedral manner. ZM sample exhibited a pronounced absorption in 370–360 nm range, accompanied by potent reflectance intensity in the Vis/NIR regions. However, Zn-MCM-41 demonstrates less reflectance intensity across the entire measured range (200–1000 nm). Notably, the introduction of either Co or Pd induces a noticeable shift towards longer wavelengths and resulting in a tail-shaped extension into the visible light spectrum. The Zn/Pd incorporation results in a decrease in diffuse reflectance intensity up to 425 nm compared to pure MCM-41. Particularly noteworthy is the significant decrease (more than 50%) in reflectance intensity upon adding 4% of Co (ZCM sample). This indicates an enhancement in the absorption of MCM-41, especially in the visible light range, upon the incorporation of Co. Previous studies showed that ZnO exhibits a wide absorption band at approximately 360 nm. This absorption is believed to be caused by charge transfer transition between O2− and Zn2+ [56, 57]. In contrast, the Zn-MCM41 samples exhibit distinct absorption bands, with peaks or shoulders at approximately 368, 276, and 246 nm, respectively. This outcome underlines the significant disparity in the way zinc species are coordinated in MCM-41 compared to pure ZnO. The band at approximately 276 nm in the ZM spectrum could be related to ZnO NPs encapsulated within the MCM-41 framework [58, 59]. The existence of an absorption peak at 246 nm is typically indicative of the inclusion of metal atoms within the MCM-41 structure, whereas the absorption band around 368 nm corresponds to the band gap width of nanocrystalline ZnO. Notably, the introduction of either cobalt or palladium (ZCM and ZPM) results in a redshift, with observed absorption bands at approximately ∼465.72 and 371.47 nm, respectively, indicating the presence of certain polymeric metal species (M–O–M) [60]. For ZCM sample, two intriguing broad absorption bands were observed at 660 nm, referring to a tetrahedrally coordinated Co ions with a 4T1(P) ← 4A transition. Simultaneously, the absorption band at 450 nm ascribed to the presence of Co species with octahedral coordination with a 4T1g(P) ← 4T1g transition [61]. This observation suggests that not all ions are enclosed within MCM-41. This correlates with the observation of finely dispersed Zn, Co and Pd particles on MCM-41-surface observed in the micrographs from scanning and transmission electron microscopy.
The band gap energy (Eg) in electron volts (eV) for the pure MCM-41 and Zn, Pd/Zn, and Co/Zn-doped samples was determined using the Tauc equation. This equation relates the measured optical absorption coefficient (α) to the photon energy (hν) and band gap energy (Eg) through the equation: \(({\upalpha }h{\upnu })^{\text{n}} = A({\text{h}}{\upnu } - {\text{E}}_{\text{g}} )\), where A is a constant, and n is either ½ or 2, indicating indirect and direct transitions, respectively. By plotting (αhν)1/2 and (αhν)2 against hν and observing their linearity, we can determine the nature of the transition and estimate band gape energy (Eg), these results are presented in Table 2 [62]. The examination of Tauc plots provides evidence that the fundamental transition in MCM-41 follows a (αhν)2 relationship with energy, suggesting that MCM-41 possesses a direct bandgap (Fig. 7b) with an energy of 5.99 eV (Fig. 7b). Kubelka − Munk function (F(R∞) was used to correlate the band gap energy (Eg) and absorption coefficients for pure, ZM, ZPM, and ZCM samples according to the equation: \(F\left( {R_\infty } \right) = \frac{K}{S} = \frac{(1 - R_\infty )^2 }{{2R_\infty }}\) where \(R_\infty = \frac{{R_{sample} }}{{R_{standard} }}\) \({\text{R}}_\infty\) represents the reflectance of an infinitely thick specimen, and S and K represent the scattering and absorption coefficients, respectively [62,63,64].
Figure 7b, c demonstrates a noteworthy reduction in the Eg upon the of Zn and Co/Zn incorporation, resulting in values of 5.86 and 5.24 eV, respectively. Conversely, a slight increase is observed with the incorporation of Pd/Zn ions (6.02 eV). Intriguingly, additional absorption bands corresponding to the Eg of ZnO and CoO are observed at 3.14, 3.18, and 1.70 eV in ZM, ZPM, and ZCM samples, respectively. The first two values closely align with the ZnO band gap [65,66,67], while the latter approximates the CoO band gap energy [68]. These findings substantiate the heightened wider wavelength-range absorption capacity of Zn, Zn/Pd, and particularly Zn/Co incorporated sample compared to the unincorporated MCM-41. The decrease in Eg is ascribed to the generation of oxygen vacancies (Vo) and the introduction new energy states through the replacing of Si4+ atoms by Zn, Pd, and Co ions. Substitution of tetravalent Si4+ with divalent (Zn2+, Pd2+, and Co2+) facilitates generation of (Vo) for charges balancing. Consequently, the Eg decline is associated with the creation of doping levels situated in-between the valence and conduction bands [69]. These results highlight the applicability of these tailored compositions for solar-photocatalysis applications.
The refractive index (n) is an important and widely used parameter in various fields, such as optics and photonics. It provides information about the behavior of light as it passes through a material, specifically how it is bent or refracted. By understanding the refractive index of a material, researchers can gain valuable perceptions of optical and potential applications in devices like waveguides and lasers. Recognized models, including the Herve-Vandamme, Moss, Sight and Reddy and Kumar models, were exploited in calculating the refractive index (n) for pure and Zn, Pd, and Co incorporating MCM-41 samples. These models are interrelated through the following relationships [70,71,72]:
Figure 7d offers a depiction of the refractive index (n) of pure-M, ZM, ZCM, and ZPM samples using the Reddy, Kumar-Singh, Moss, and Herve-Vandamme models. The Reddy, Kumar-Singh, and Moss models have shown agreement in their calculated (n) values, close to 2. Following the Reddy, Kumar-Singh, Moss, and Herve-Vandamme models, the MCM-41 sample exhibits refractive index of 2.28846, 1.89171, 1.99644, and 1.75247, respectively. The addition of Zn, Co/Zn, or Pd/Zn ions results in a clear enhancement in (n). This increase is particularly noticeable in the ZCM sample, as stated in Table 2. Such refractive index improvement could be assigned to the generation of Vo as result of the incorporation of Zn2+, Pd2+, and Co2+ ions (Eqs. 1–3). These findings go consistent with previous reports demonstrating similar trends of refractive index enhancement in SiO2 associated with the presence of oxygen vacancies [73].
The intentional introduction of Zn, Zn/Co and Zn/Pd dopants caused a decrease the Eg and an increase in the refractive index of the MCM-41. This has significant implications for various applications, such as photocatalysis and optoelectronics, as the doped samples exhibit improved performance in these areas.
3.6 Infrared spectra
Figure 8a illustrates the infrared spectra of both pure and Zn, Zn/Co and Zn/Pd-MCM-41 samples; with a magnified incent for the region 400–1400 cm−1 (Fig. 8b). The observed bands within the 400–4000 cm−1 range are characteristic of the vibrational modes associated with the MCM-41 structural network [74, 75].
The pure-M sample showed a broad absorption band at 3380.30 cm−1 credited to υstretching of (SiOH) groups, perturbed by stretching H2Oads [76, 77]. Additionally, the 1634.47 cm−1 peak matches to the υbending of H2Oads. Detected peaks at 1229.82 and 1062.38 cm−1 are associated with the υstretching of the silicon-oxygen-silicon bonds, whereas the 963.89 cm−1 band is owed to stretching vibrations of silicon-oxygen-hydrogen (υass(SiOH)) bonds. The transmission at 796.44 cm−1 is associated with the υstretching of silicon-oxygen-silicon bonds, and the distinct peak at 447.61 cm−1 indicates υbending of siloxane (δ(Si–O-Si)) bonds. These findings hold true for the ZM, ZCM, and ZPM samples (Fig. 8), the transmittance intensity of the 3380.30 and 1634.47 cm−1 bands of pure MCM-41 decreases, with a slight shift towards lower wavenumbers. While the band at 1634.47 cm−1 remains unchanged for the ZPM sample, the shifts in the other bands are considered indicative of the inclusion of metal into the MCM-41 pores, which aligns with previous studies [78, 79]. A shifting in 1062.38 cm−1 band to 1054.99 cm−1 was noticed for both ZM and ZCM samples, with no shift observed for the ZPM sample. Additionally, an intensity altering in the 963.89 cm−1 peak is detected upon metal incorporation, which signifies a modification in the Si–OH surface groups upon M-doping. Previous studies have also shown that the inclusion of zinc and cerium ions resulted in similar effects [80, 81]. The detected variations of silanol groups intensity serves as proof of the successful integration of metal into the MCM-41 structure [82]. Additionally, finite bands at 580.57 and 594.53 cm−1 appear after introducing cobalt and palladium atoms, respectively, into MCM-41; These bands could be are assigned to Si–O–(Pd/Co) species.
3.7 Antimicrobial activity
The bactericidal efficacy of both pristine MCM-41 as well as Zn, Zn/Co and Zn/Pd nanocompositions, in comparison to a commercially available antibiotic (used as the control sample), was investigated against Gram-positive bacteria strains, Gram-negative bacteria strains, and yeast strains. The results, presented in Table 3 and Fig. 9 emphasize the superior antimicrobial activity exhibited by metal incorporated MCM-41 compounds against the diverse range of tested microorganisms. However, when it comes to the pure-M, it did not exhibit any activity showed no action against any of the tested spectrum of microorganisms. This heightened antimicrobial performance is attributed to the specific compounds loaded onto the MCM-41 matrix.
Among the tested compounds, ZM demonstrated the highest activity against bacteria Bacillus cereus and Pseudomonas aeruginosa, comparable to the reference antibiotic. Following closely, compound ZPM also exhibited potent antimicrobial effects. Conversely, in the context of inhibiting Gram-negative bacteria Salmonella typhimurium, ZM displayed the most pronounced inhibitory effect, surpassing the reference antibiotic. Additionally, ZPM demonstrated noteworthy inhibitory activity against Escherichia coli, surpassing the Ampicillin and very close to reference drug against Bacillus cereus, Pseudomonas aeruginosa, Salmonella typhimurium. Also, ZCM showed potent activity towards Escherichia coli, Salmonella typhimurium. This high activity could be related to that the combination of MCM-41 and metal ions and/or two different metal ions may result in synergistic effects, enhancing the overall antimicrobial activity. Synergies between metal ions or metal ions and MCM-41 can lead to increased efficacy against a broader range of microorganisms.
The observed superior antimicrobial activity of the metal ions incorporated MCM-41 nanocomposite can be attributed to their higher specific surface area compared to the commercial drugs. The well-established relationship between surface area and antimicrobial activity, documented in the literature, supports the notion that the enhanced surface area of nanocomposite contributes to its superior performance [83, 84]. The more acidic active sites induced in MCM-41 upon metal incorporation can create an environment that is hostile to bacteria and fungi through donation protons to biomolecules, disrupting their structure and function, further contributing to their potent antimicrobial effects. Additionally, enhanced electrostatic interactions between the nanocomposites and microorganisms act a crucial part. These interactions lead to changes in the morphology of microbial membranes, inducing disruptions that ultimately result in cell death [85]. The existence of Zn, Co, and Pd, in the nanocomposites can also have direct bactericidal effects. Theses metal ions have the potential to disturb the membranes of microbial cells, interfere with crucial cellular functions, which generate an oxidative stress and ultimately resulting in the inhibition of bacterial growth [86,87,88,89,90]. This multifaceted approach, combining surface area advantages and electrostatic interactions, underscores the effectiveness of ZM, ZCM and ZPM against a broad spectrum of microorganisms.
These findings highlight the remarkable antimicrobial potential of the studied compounds, demonstrating their ability to combat a wide spectrum of bacteria and yeast. The Antimicrobial activities of the synthesized compounds compared to some compounds reported in the literature are listed in Table 4. The varied antimicrobial activities observed are attributed to the distinct compositions loaded onto the MCM-41, opening avenues for the development of antimicrobial agents with tailored efficacy against specific microorganisms.
4 Conclusion
A series of mesoporous materials, including highly ordered pure MCM-41, mono-metallic Zn, and bimetallic Zn/Co- and Zn/Pd-MCM-41, were successfully synthesized using a surfactant-assisted precipitation method. Characterization through various techniques confirmed the structural and morphological properties of these materials. The metal incorporation was validated by XRD and instigated a reduction in specific surface area as a result of partial filling of mesopores. The optical properties revealed a decrease in the band gap upon metal incorporation, making the compounds promising for diverse applications, particularly in optoelectronics and visible light photocatalysis. Furthermore, the antimicrobial activity of the synthesized materials was explored, with the ZM compound exhibiting the most substantial inhibitory performance towards both Gram-positive and Gram-negative bacteria. Notably, ZPM demonstrated significant inhibitory activity against Gram-negative bacteria, surpassing Ampicillin antibiotic. Incorporation of Zn, Co and Pd bring about partial alterations of the mesophase structure with a reduced surface area. Yet, the more acidic active sites presented by these metal species, significantly contributed to the high antimicrobial activity. These findings emphasize the potential of metal-incorporated compounds, especially ZM, ZPM, and ZCM, as promising antimicrobial agents with applications spanning various fields.
Data and code availability
Data will be made available on request.
References
I.I. Slowing, J.L. Vivero-Escoto, B.G. Trewyn, V.S.-Y. Lin, Mesoporous silica nanoparticles: structural design and applications. J. Mater. Chem. 20(37), 7924–7937 (2010)
W.E. Rashwan, K.S. Abou-El-Sherbini, M.A. Wahba, S.A. Sayed Ahmed, P.G. Weidler, High stable Al-MCM-41: structural characterization and evaluation for removal of methylene blue from aqueous solution. SILICON 12, 2017–2029 (2020)
B. Matkala, S. Boggala, S. Basavaraju, V.S. Akella, H.P. Aytam, Influence of sulphonation on Al-MCM-41 catalyst for effective bio-glycerol conversion to Solketal. Microporous Mesoporous Mater. 363, 112830 (2024)
A.G. Gebretatios, F. Banat, T. Witoon, C.K. Cheng, Synthesis of sustainable rice husk ash-derived nickel-decorated MCM-41 and SBA-15 mesoporous silica materials for hydrogen storage. Int. J. Hydrogen Energy 51, 255–266 (2024)
T.T.H. Thi, T.N.Q. Nguyen, D.T. Hoang, D.H. Nguyen, Functionalized mesoporous silica nanoparticles and biomedical applications. Mater. Sci. Eng. C 99, 631–656 (2019)
S. Jafari, H. Derakhshankhah, L. Alaei, A. Fattahi, B.S. Varnamkhasti, A.A. Saboury, Mesoporous silica nanoparticles for therapeutic/diagnostic applications. Biomed. Pharmacother. 109, 1100–1111 (2019)
R.K. Kankala, Y.H. Han, J. Na, C.H. Lee, Z. Sun, S.B. Wang, T. Kimura, Y.S. Ok, Y. Yamauchi, A.Z. Chen, Nanoarchitectured structure and surface biofunctionality of mesoporous silica nanoparticles. Adv. Mater. 32(23), 1907035 (2020)
D. Khan, and Shaily, Synthesis and catalytic applications of organo-functionalized MCM-41 catalyst: A review. Appl. Organomet. Chem. 37(3), e7007 (2023)
W. Henao, L. Jaramillo, D. López, M. Romero-Sáez, R. Buitrago-Sierra, Insights into the CO2 capture over amine-functionalized mesoporous silica adsorbents derived from rice husk ash. J. Environ. Chem. Eng. 8(5), 104362 (2020)
N.H.M.H. Tehrani, M. Ardjmand, M. Bazmi, A. Rashidi, H.R.M. Zadeh, Polydopamine-modified mesoporous silica materials as a novel adsorbent for superior CO2 adsorption: experimental and DFT study. J. Environ. Chem. Eng. 11(5), 110451 (2023)
S. Sohrabnezhad, A. Valipour, Synthesis of Cu/CuO nanoparticles in mesoporous material by solid state reaction. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 114, 298–302 (2013)
J.A.S. Costa, R.A. de Jesus, D.O. Santos, J.F. Mano, L.P. Romao, C.M. Paranhos, Recent progresses in the adsorption of organic, inorganic, and gas compounds by MCM-41-based mesoporous materials. Microporous Mesoporous Mater. 291, 109698 (2020)
C. Wu, Y. Li, Y. Ma, Y. Lei, M. Wang, Z. Chen, Synthesis of CuI-modified thiol-functionalized MCM-41 silica-based materials and their catalytic tetralin performance. J. Porous Mater. 31, 913–922 (2024)
R.K. Khaled, M.A. Wahba, M.D. Badry, M. Zawrah, E. Heikal, Highly ordered pure and indium-incorporated MCM-41 mesoporous adsorbents: synthesis, characterization and evaluation for dye removal. J. Mater. Sci. 57(7), 4504–4527 (2022)
X. Yu, C.T. Williams, Recent advances in the applications of mesoporous silica in heterogeneous catalysis. Catal. Sci. Technol. 12(19), 5765–5794 (2022)
W. Han, S. Qiu, J. Chen, X. Zhong, L. Hao, H. Chen, X. Zhou, H. Zhou, One-pot synthesis of mesoporous silica-supported nano-metal oxide composites with enhanced antibacterial properties. Mater. Chem. Phys. 290, 126618 (2022)
M.L. Saladino, E. Kraleva, S. Todorova, A. Spinella, G. Nasillo, E. Caponetti, Synthesis and characterization of mesoporous Mn-MCM-41 materials. J. Alloys Compd. 509(35), 8798–8803 (2011)
J. Zhao, Y. Zhang, L. Xu, F. Tian, T. Hu, C. Meng, Weak base favoring the synthesis of highly ordered V-MCM-41 with well-dispersed vanadium and the catalytic performances on selective oxidation of benzyl alcohol. Chin. J. Chem. Eng. 28(5), 1424–1435 (2020)
J. Grzybek, B. Gil, W.J. Roth, M. Skoczek, A. Kowalczyk, L. Chmielarz, Characterization of Co and Fe-MCM-56 catalysts for NH3-SCR and N2O decomposition: an in situ FTIR study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 196, 281–288 (2018)
K. Katok, V. Tertykh, S.Y. Brichka, G. Prikhod’ko, Catalytic synthesis of carbon nanotubes over ordered mesoporous matrices. J. Therm. Anal. Calorim. 86, 109–114 (2006)
M. Kruk, Access to ultralarge-pore ordered mesoporous materials through selection of surfactant/swelling-agent micellar templates. Acc. Chem. Res. 45(10), 1678–1687 (2012)
R. Kumar, S. Shah, J. Bahadur, Y.B. Melnichenko, D. Sen, S. Mazumder, C.P. Vinod, B. Chowdhury, Highly stable In-SBA-15 catalyst for vapor phase Beckmann rearrangement reaction. Microporous Mesoporous Mater. 234, 293–302 (2016)
S. Jabariyan, M.A. Zanjanchi, M. Arvand, S. Sohrabnezhad, Colorimetric detection of glucose using lanthanum-incorporated MCM-41. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 203, 294–300 (2018)
V. Pârvulescu, C. Tablet, C. Anastasescu, B. Su, Activity and stability of bimetallic Co (V, Nb, La)-modified MCM-41 catalysts. Catal. Today 93, 307–313 (2004)
Guthrie, C.P., Behaviour of mesoporous silica (MCM-41) supported catalysts in degradation reactions (2013)
P. Decyk, States of transition metal ions in modified mesoporous MCM-41 and in microporous ZSM-5 studied by ESR spectroscopy. Catal. Today 114(2–3), 142–153 (2006)
F. Habeche, B. Boukoussa, I. Issam, A. Mokhtar, X. Lu, J. Iqbal, S. Hacini, M. Hachemaoui, A. Bengueddach, R. Hamacha, Catalytic reduction of organic pollutants, antibacterial and antifungal activities of AgNPs@ CuO nanoparticles–loaded mesoporous silica. Environ. Sci. Pollut. Res. 30(11), 30855–30873 (2023)
N.A. Atiyah, M.A. Atiya, T.M. Albayati, Curcumin loaded onto magnetic mesoporous material MCM-41 for controlled and released in drug delivery system. Eng. Technol. J. 40(3), 472–483 (2022)
X. Cao, T. Ai, Z. Xu, J. Lu, D. Chen, D. He, J. Liu, R. Tian, Y. Zhao, Y. Luo, Insights into the different catalytic behavior between Ce and Cr modified MCM-41 catalysts: Cr2S3 as new active species for CH3SH decomposition. Sep. Purif. Technol. 307, 122742 (2023)
L. Huang, F. Tang, F. Hao, H. Zhao, W. Liu, Y. Lv, P. Liu, W. Xiong, H. Luo, Tuning the electron density of metal nickel via interfacial electron transfer in Ni/MCM-41 for efficient and selective catalytic hydrogenation of halogenated nitroarenes. ACS Sustain. Chem. Eng. 10(9), 2947–2959 (2022)
M.A. Shahzad, H. Khan, A.A. Mangi, R. Ashraf, E. Dar, Synthesis, characterization, and antibacterial activity of metal doped copper oxide MCM-41 nanocomposites. J. Hunan Univ. Nat. Sci. 49, 2 (2022). https://doi.org/10.55463/issn.1674-2974.49.2.28
C.-H. Wang, Y.-H. Hsieh, Z.M. Powers, C.-Y. Kao, Defeating antibiotic-resistant bacteria: exploring alternative therapies for a post-antibiotic era. Int. J. Mol. Sci. 21(3), 1061 (2020)
N. Oliveira, B. Gonçalves, S. Lee, C. Oliveira, C. Corassin, Use of antibiotics in animal production and its impact on human health. J. Food Chem. Nanotechnol. 6(01), 40–47 (2020)
M.R. Abukhadra, W. Fathallah, F.A. El Kashief, A.M. El-Sherbeeny, M.A. El-Meligy, E.M. Awwad, M. Luqman, Insight into the antimicrobial and photocatalytic properties of NiO impregnated MCM-48 for effective removal of pathogenic bacteria and toxic levofloxacin residuals. Microporous Mesoporous Mater. 312, 110769 (2021)
S. Joardar, M.L. Adams, R. Biswas, G.V. Deodhar, K.E. Metzger, K. Deweese, M. Davidson, R.M. Richards, B.G. Trewyn, P. Biswas, Direct synthesis of silver nanoparticles modified spherical mesoporous silica as efficient antibacterial materials. Microporous Mesoporous Mater. 313, 110824 (2021)
B. Asli, S. Abdelkrim, M. Zahraoui, A. Mokhtar, M. Hachemaoui, F. Bennabi, A.B. Ahmed, A. Sardi, B. Boukoussa, Catalytic reduction and antibacterial activity of MCM-41 modified by silver nanoparticles. SILICON 14(18), 12587–12598 (2022)
V.-E. Copcia, R. Gradinaru, G.D. Mihai, N. Bilba, I. Sandu, Antibacterial activity of nanosized ZnO hosted in microporous clinoptilolite and mesoporous silica SBA-15 matrices. Rev. De Chim. 63(11), 1124–1131 (2012)
A. Donnadio, G. Cardinali, L. Latterini, L. Roscini, V. Ambrogi, Nanostructured zinc oxide on silica surface: Preparation, physicochemical characterization and antimicrobial activity. Mater. Sci. Eng. C 104, 109977 (2019)
S. Nazir, K. Tahir, R. Irshad, Q.U. Khan, S. Khan, I.U. Khan, A. Nawaz, F. Rehman, Photo-assisted inactivation of highly drug resistant bacteria and DPPH scavenging activities of zinc oxide graphted Pd-MCM-41 synthesized by new hydrothermal method. Photodiagn. Photodyn. Ther. 33, 102162 (2021)
F. Mansour, R. Dimeo, H. Peemoeller, High-resolution inelastic neutron scattering from water in mesoporous silica. Phys. Rev. E 66(4), 041307 (2002)
C.-F. Cheng, H. He, W. Zhou, J. Klinowski, J.A.S. Gonçalves, L.F. Gladden, Synthesis and characterization of the gallosilicate mesoporous molecular sieve MCM-41. J. Phys. Chem. 100(1), 390–396 (1996)
T. Takeguchi, J.-B. Kim, M. Kang, T. Inui, W.-T. Cheuh, G.L. Haller, Synthesis and characterization of alkali-free, Ga-substituted MCM-41 and its performance for n-hexane conversion. J. Catal. 175(1), 1–6 (1998)
Q. Wang, S. Zhang, Y. Yu, B. Dai, High-performance of plasma-enhanced Zn/MCM-41 catalyst for acetylene hydration. Catal. Commun. 147, 106122 (2020)
M. Kruk, M. Jaroniec, Y. Sakamoto, O. Terasaki, R. Ryoo, C.H. Ko, Determination of pore size and pore wall structure of MCM-41 by using nitrogen adsorption, transmission electron microscopy, and X-ray diffraction. J. Phys. Chem. B 104(2), 292–301 (2000)
M. Kruk, M. Jaroniec, A. Sayari, Relations between pore structure parameters and their implications for characterization of MCM-41 using gas adsorption and X-ray diffraction. Chem. Mater. 11(2), 492–500 (1999)
S. Suvanto, J. Hukkamäki, T. Pakkanen, T. Pakkanen, High-cobalt-loaded MCM-41 via the gas-phase method. Langmuir 16(9), 4109–4115 (2000)
Z. Liu, R. Zhou, X. Zheng, Comparative study of different methods of preparing CuO–CeO2 catalysts for preferential oxidation of CO in excess hydrogen. J. Mol. Catal. A Chem. 267(1–2), 137–142 (2007)
P.-O. Larsson, A. Andersson, Complete oxidation of CO, ethanol, and ethyl acetate over copper oxide supported on titania and ceria modified titania. J. Catal. 179(1), 72–89 (1998)
T. Sun, T. Lei, Z. Li, Z. Zhang, S. Yang, X. Xin, M. Zhang, X. He, Q. Zhang, L. Zhang, Catalytic co-pyrolysis of corn stalk and polypropylene over Zn–Al modified MCM-41 catalysts for aromatic hydrocarbon-rich oil production. Ind. Crops Prod. 171, 113843 (2021)
G. Su, S. Qiu, J. Lin, X. Zhong, H. Zhou, X. Zhou, Mesoporous silica doped with different water-soluble ligands to enhance the antibacterial performance of nano zinc oxides by coordination effect. Colloids Surf. A 640, 128414 (2022)
Y. Gucbilmez, T. Dogu, S. Balci, Vanadium incorporated high surface area MCM-41 catalysts. Catal. Today 100(3), 473–477 (2005)
R. Kumar Rana, B. Viswanathan, Mo incorporation in MCM-41 type zeolite. Catal. Lett. 52, 25–29 (1998)
W. Zhang, M. Fröba, J. Wang, P.T. Tanev, J. Wong, T.J. Pinnavaia, Mesoporous titanosilicate molecular sieves prepared at ambient temperature by electrostatic (S+ I-, S+ X-I+) and neutral (S° I°) assembly pathways: a comparison of physical properties and catalytic activity for peroxide oxidations. J. Am. Chem. Soc. 118(38), 9164–9171 (1996)
C. Dong, X. Li, A. Wang, Y. Chen, H. Liu, Influence of nanoscale distribution of Pd particles in the mesopores of MCM-41 on the catalytic performance of Pd/MCM-41. Catal. Commun. 100, 219–222 (2017)
S. Bhattacharyya, G. Lelong, M.-L. Saboungi, Recent progress in the synthesis and selected applications of MCM-41: a short review. J. Exp. Nanosci. 1(3), 375–395 (2006)
S. Bordiga, C. Lamberti, G. Ricchiardi, L. Regli, F. Bonino, A. Damin, K.-P. Lillerud, M. Bjorgen, A. Zecchina, Electronic and vibrational properties of a MOF-5 metal–organic framework: ZnO quantum dot behaviour. Chem. Commun. 20, 2300–2301 (2004)
C. Cristiani, P. Forzatti, M. Bellotto, Structural investigation on a spinel-related Zn/Cr= 1 mixed-oxide system. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 85(4), 895–906 (1989)
E.A. Alarcón, A.L. Villa, C.M. de Correa, Characterization of Sn-and Zn-loaded MCM-41 catalysts for nopol synthesis. Microporous Mesoporous Mater. 122(1–3), 208–215 (2009)
L. Wang, S. Sang, S. Meng, Y. Zhang, Y. Qi, Z. Liu, Direct synthesis of Zn-ZSM-5 with novel morphology. Mater. Lett. 61(8–9), 1675–1678 (2007)
Y. Hu, Y. Nagai, D. Rahmawaty, C. Wei, M. Anpo, Characteristics of the photocatalytic oxidation of methane into methanol on V-containing MCM-41 catalysts. Catal. Lett. 124(1), 80–84 (2008)
C. He, M. Paulus, W. Chu, J. Find, J.A. Nickl, K. Köhler, Selective catalytic reduction of NO by C3H8 over CoOx/Al2O3: An investigation of structure–activity relationships. Catal. Today 131(1–4), 305–313 (2008)
P. Makuła, M. Pacia, W. Macyk, How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV–Vis spectra. J. Phys. Chem. Lett. 9, 6814–6817 (2018)
M.A. Wahba, W. Sharmoukh, S.M. Yakout, M.S. Khalil, Fast and full spectrum sunlight photocatalysts: Fe/Co or Ni implanted multiferroic LaMnO3. Opt. Mater. 124, 111973 (2022)
M.A. Wahba, Visible-light responsive BiVO4 nanocompositions: Enhanced photocatalytic, electrical and optical performance through Ni and Ni/Co doping. Opt. Mater. 147, 114643 (2024)
M.A. Wahba, S.M. Yakout, W.A. Mohamed, H.R. Galal, Remarkable photocatalytic activity of Zr doped ZnO and ZrO2/ZnO nanocomposites: Structural, morphological and photoluminescence properties. Mater. Chem. Phys. 256, 123754 (2020)
M.A. Wahba, S.M. Yakout, R. Khaled, Interface engineered efficient visible light photocatalytic activity of MWCNTs/Co doped ZnO nanocomposites: morphological, optical, electrical and magnetic properties. Opt. Mater. 115, 111039 (2021)
M.A. Wahba, S.M. Yakout, Microwave-synthesized ZrO2/ZnO heterostructures: fast and high charge separation solar catalysts for dyes-waste degradation. J. Sol-Gel Sci. Technol. 104(2), 330–341 (2022)
S.N.F. Moridon, M.I. Salehmin, M.A. Mohamed, K. Arifin, L.J. Minggu, M.B. Kassim, Cobalt oxide as photocatalyst for water splitting: temperature-dependent phase structures. Int. J. Hydrogen Energy 44(47), 25495–25504 (2019)
Y. Yuan, Y. Huang, F. Ma, Z. Zhang, X. Wei, Effects of oxygen vacancy on the mechanical, electronic and optical properties of monoclinic BiVO4. J. Mater. Sci. 52(14), 8546–8555 (2017)
S. Senol, E. Ozugurlu, L. Arda, Synthesis, structure and optical properties of (Mn/Cu) co-doped ZnO nanoparticles. J. Alloys Compd. 822, 153514 (2020)
S. Senol, B. Yalcin, E. Ozugurlu, L. Arda, Structure, microstructure, optical and photocatalytic properties of Mn-doped ZnO nanoparticles. Mater. Res. Express 7(1), 015079 (2020)
S. Tripathy, Refractive indices of semiconductors from energy gaps. Opt. Mater. 46, 240–246 (2015)
F. Ehré, C. Labbé, C. Dufour, W. Jadwisienczak, J. Weimmerskirch-Aubatin, X. Portier, J.-L. Doualan, J. Cardin, A. Richard, D. Ingram, The nitrogen concentration effect on Ce doped SiO x N y emission: towards optimized Ce 3+ for LED applications. Nanoscale 10(8), 3823–3837 (2018)
G.-N. Yun, S.-J. Ahn, A. Takagaki, R. Kikuchi, S.T. Oyama, Infrared spectroscopic studies of the hydrodeoxygenation of γ-valerolactone on Ni2P/MCM-41. Catal. Today 323, 54–61 (2019)
M.G. Miricioiu, C. Iacob, G. Nechifor, V.-C. Niculescu, High selective mixed membranes based on mesoporous MCM-41 and MCM-41-NH2 particles in a polysulfone matrix. Front. Chem. 7, 332 (2019)
D. Xie, Y. Jiang, Y. Zhang, B. Song, Salt-resistant switchable pickering emulsions stabilized by mesoporous nanosilica hydrophobized in situ by pH-insensitive surfactants. Langmuir 37(19), 5846–5853 (2021)
S. Parasuraman, K. Suresh, G. Chandrashekar, Catalytic wet air oxidation of aniline on transition metal modified MCM-41 catalysts. Ind. Eng. Chem. Res. 40, 3237–3261 (2001)
N.K. Mal, P. Kumar, M. Fujiwara, K. Kuraoka, Restructured V-MCM-41 with non-leaching vanadium and improved hydrothermal stability prepared by secondary synthesis, in Studies in Surface Science and Catalysis. (Elsevier, 2002), pp.1307–1314
N.K. Mal, V. Ramaswamy, S. Ganapathy, A. Ramaswamy, Synthesis and characterization of crystalline, tin-silicate molecular sieves with MFI structure. J. Chem. Soc. Chem. Commun. 17, 1933–1934 (1994)
D. Yin, L. Qin, J. Liu, C. Li, Y. Jin, Gold nanoparticles deposited on mesoporous alumina for epoxidation of styrene: Effects of the surface basicity of the supports. J. Mol. Catal. A Chem. 240(1–2), 40–48 (2005)
K.M.S. Khalil, Cerium modified MCM-41 nanocomposite materials via a nonhydrothermal direct method at room temperature. J. Colloid Interface Sci. 315(2), 562–568 (2007)
F. Raji, M. Pakizeh, Study of Hg(II) species removal from aqueous solution using hybrid ZnCl2-MCM-41 adsorbent. Appl. Surf. Sci. 282, 415–424 (2013)
A.J. Cunliffe, P.D. Askew, I. Stephan, G. Iredale, P. Cosemans, L.M. Simmons, J. Verran, J. Redfern, How do we determine the efficacy of an antibacterial surface? A review of standardised antibacterial material testing methods. Antibiotics 10(9), 1069 (2021)
U. Mahanta, M. Khandelwal, A.S. Deshpande, Antimicrobial surfaces: a review of synthetic approaches, applicability and outlook. J. Mater. Sci. 56(32), 17915–17941 (2021)
A. Pugazhendhi, D. Prabakar, J.M. Jacob, I. Karuppusamy, R.G. Saratale, Synthesis and characterization of silver nanoparticles using Gelidium amansii and its antimicrobial property against various pathogenic bacteria. Microb. Pathog. 114, 41–45 (2018)
M. Godoy-Gallardo, U. Eckhard, L.M. Delgado, Y.J. de Roo Puente, M. Hoyos-Nogués, F.J. Gil, R.A. Perez, Antibacterial approaches in tissue engineering using metal ions and nanoparticles: From mechanisms to applications. Bioact. Mater. 6(12), 4470–4490 (2021)
K. Gold, B. Slay, M. Knackstedt, A.K. Gaharwar, Antimicrobial activity of metal and metal-oxide based nanoparticles. Adv. Ther. 1(3), 1700033 (2018)
A. El-Tabl, M. Wahed, M. Wahba, S. El-assaly, L. Saad, sugar hydrazone complexes; synthesis, spectroscopic characterization and antitumor activity. J. Adv. Chem. 9, 1837 (1860)
A.S. El-Tabl, M.M. Abd-El Wahed, M.A. Wahba, M. Shakdofa, A. Gafer, Bimetallic transition metal complexes of 2,3-dihydroxy-N′,N′4-bis ((2-Hydroxynaphthalen-1-yl) methylene) succinohydrazide ligand as a new class of bioactive compounds; synthesis, characterization and cytotoxic evaluation. Indian J. Adv. Chem. Sci. 4(1), 114–129 (2016)
P. More, V. Inamdar, S. Suresh, S. Dindorkar, S. Peddakolmi, K. Jain, N. Khona, S. Khatoon, S. Patange, Synthesis of zinc oxide nanoparticles using Chrysopogonzizanioides grass extract, its applications in photodegradation and antimicrobial activity. J. Mater. Sci. Mater. Electron. 32(15), 20725–20741 (2021)
I.M. El Nahhal, H.H. Almutairi, J.K. Salim, F.S. Kodeh, R.H. Idais, ZnO-NPs/AC composite antibacterial agents with N-halamine glycinate functionalized silica-mesoporous silica coating for water disinfection. Heliyon 10(2), e24343 (2024)
P. Pongchaikul, T. Hajidariyor, N. Khetlai, Y.-S. Yu, P. Arjfuk, P. Khemthong, W. Wanmolee, P. Posoknistakul, N. Laosiripojana, K.C.-W. Wu, Nanostructured N/S doped carbon dots/mesoporous silica nanoparticles and PVA composite hydrogel fabrication for anti-microbial and anti-biofilm application. Int. J. Pharm. X 6, 100209 (2023)
L. Wang, H. He, C. Zhang, L. Sun, S. Liu, R. Yue, Excellent antimicrobial properties of silver-loaded mesoporous silica SBA-15. J. Appl. Microbiol. 116(5), 1106–1118 (2014)
D.-D. Zhang, S. Hu, P.-Y. Pan, M. Zhang, Q. Wu, Y.-R. Zhang, X.-Q. Zhou, Preparation and antibacterial activity of mesoporous silica based on rice husk ash. LWT 185, 115145 (2023)
H.T. Trinh, T.K.A. Tran, S. Arora, S.M. George, J. Sheri, Z. Li, J.H. Yang, P. Naruphontjirakul, K. Balani, A. Karakoti, Zn-Loaded SBA-1 and SBA-15 molecular sieves for combined antimicrobial and osteogenic activity. Adv. Mater. Technol. 8(6), 2201169 (2023)
G.A. Marcelo, M.P. Duarte, E. Oliveira, Gold@ mesoporous silica nanocarriers for the effective delivery of antibiotics and by-passing of β-lactam resistance. SN Appl. Sci. 2, 1–15 (2020)
L. Tahmasbi, T. Sedaghat, H. Motamedi, Synthesis of novel mesoporous silica nanoparticles functionalized with succinic dihydrazone Schiff-base metal complexes and a study of their biological activities. Mater. Adv. 4(13), 2770–2779 (2023)
Acknowledgements
This study was funded by the National Research Centre, project number (12020224), principal researcher: Professor Magda D. Badri.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Mohammed Wahba: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing–original draft, Review& editing. Rabab Khaled: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing original draft, Review & editing. Magda Badry: Conceptualization, Validation, Formal analysis, Investigation, Data curation, Review & editing. Maysa Moharam: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – original draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wahba, M.A., Khaled, R.K., Dawy, M. et al. Enhanced optical and antimicrobial activities of mono Zn and bimetallic (Zn, Co), (Zn, Pd) ions modified MCM-41: structural and morphological investigation. J Porous Mater (2024). https://doi.org/10.1007/s10934-024-01634-4
Published:
DOI: https://doi.org/10.1007/s10934-024-01634-4