Abstract
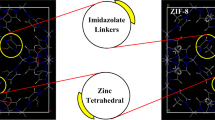
Zeolitic imidazolate frameworks (ZIFs) possess similar topological structures with zeolites, however a number of characteristic properties of the ZIFs, such as easy incorporation of desired linkers to their structures, tunable pore sizes, high porosities, and large surface areas make them better option for targeted engineering applications. In this respect, cheaper, highly efficient, and selective separation of syngas mixtures using ZIFs has been already shown in the literature. Separation and purification of noble gases, on the other hand, have been achieved using energy demanding cryogenic distillation of air and currently we know little about noble gas separation performances of the ZIFs. Replacing energy intensive gas separation techniques with adsorbent and/or membrane based separation technologies, indeed, have been motivated for decreasing the operational costs, price of the pure noble gases, as well as opening new application areas for the noble gases. In this respect, we theoretically investigate Xe/Kr and Xe/Ar separation performances of eight ZIFs, namely ZIF-6, ZIF-11, ZIF-60, ZIF-67, ZIF-69, ZIF-78, ZIF-79, and ZIF-81 using Grand Canonical Monte Carlo and Molecular Dynamics simulations and calculate Xe permeability and permeation selectivity using a newer method suggested previously by our group. While ZIF-11 shows significant Xe uptake from the noble gas mixtures, ZIF-81 and ZIF-79 show exceptional Xe adsorption selectivities for two gas mixtures at low pressures, 22.5 and 21 for the Xe/Kr and 128 and 120 for the Xe/Ar mixture, respectively. ZIF-69 and ZIF-79 show high Xe permeability (4.\(2\times 1\)0\(^5\) and 3.\(2\times 1\)0\(^5\) Barrers) as well as high Xe permeation selectivity (17.35 and 15.85) for the Xe/Ar mixture at 2 bar feed gas pressure. For the Xe/Kr mixture, on the other hand, we observe high Xe permeability and moderate Xe permeation selectivity, especially at low pressures. Comparing with the limited literature available, these ZIFs show promise for selective separation of Xe from its binary mixtures of Kr and Ar at room temperature. We also compare the permeability and permeation selectivity predictions using different approaches, namely the new method and the approximate approach which is commonly used in the literature. Results reveal significant deviations of the approximate approach with respect to the new method, especially for the permeability calculations. Thus, using the new method for determining membrane performances is highly suggested.




Similar content being viewed by others
References
P. Kah, J. Martikainen, Influence of shielding gases in the welding of metals. Int. J. Adv. Manuf. Technol. 64, 1411–1421 (2013)
D. Banerjee, C.M. Simon, S.K. Elsaidi, M. Haranczyk, P.K. Thallapally, Xenon gas separation and storage using metal-organic frameworks. Chemistry 4, 466–494 (2018)
A.E. Neice, M.H. Zornow, Xenon anaesthesia for all, or only a select few? Anaesthesia 71, 1267–1272 (2016)
S.K. Elsaidi, D. Ongari, W. Xu, M.H. Mohamed, M. Haranczyk, P.K. Thallapally, Xenon recovery at room temperature using metal-organic frameworks. Chemistry A 23, 10758–10762 (2017)
Z. Sumer, S. Keskin, Molecular simulations of mof adsorbents and membranes for noble gas separations. Chem. Eng. Sci. 164, 108–121 (2017)
B. Chen, Z. Yang, Y. Zhu, Y. Xia, Zeolitic imidazolate framework materials: recent progress in synthesis and applications. J. Mater. Chem. A 2, 16811–16831 (2014)
H. Wang, J. Li, General strategies for effective capture and separation of noble gases by metal-organic frameworks. Dalton Trans. 47, 4027–4031 (2018)
H. Li, K. Haas-Santo, U. Schygulla, R. Dittmeyer, Inorganic microporous membranes for H2 and CO2 separation-Review of experimental and modeling progress. Chem. Eng. Sci. 127, 401–417 (2015)
T. Barton, L. Bull, W. Klemperer, D. Loy, B. McEnaney, M. Misono, P. Monson, G. Pez, G. Scherer, J. Vartuli, O. Yaghi, Tailored porous materials. Chem. Mater. 11, 2633–2656 (1999)
A. Cheetham, G. Ferey, T. Loiseau, Open-framework inorganic materials. Angewandte Chemie-International Edition 38, 3268–3292 (1999)
O. Yaghi, G. Li, H. Li, Selective Binding and removal of guests in a microporous metal-organic Framework. Nature 378, 703–706 (1995)
E. Adatoz, A.K. Avci, S. Keskin, Opportunities and challenges of MOF-based membranes in gas separations. Sep. Purif. Technol. 152, 207–237 (2015)
K.S. Park, Z. Ni, A.P. Côté, J.Y. Choi, R. Huang, F.J. Uribe-Romo, H.K. Chae, M. O’Keeffe, O.M. Yaghi, Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Nat. Acad. Sci. U.S.A. 103, 10186–10191 (2006)
R. Banerjee, A. Phan, B. Wang, C. Knobler, H. Furukawa, M. O’Keeffe, O.M. Yaghi, High-throughput synthesis of zeolitic imidazolate frameworks and application to co2 capture. Science 319, 939–943 (2008)
R. Grau-Crespo, A. Aziz, A.W. Collins, R. Crespo-Otero, N.C. Hernández, L.M. Rodriguez-Albelo, A.R. Ruiz-Salvador, S. Calero, S. Hamad, Modelling a linker mix-and-match approach for controlling the optical excitation gaps and band alignment of zeolitic imidazolate frameworks. Angew. Chem. Int. Ed. 55, 16012–16016 (2016)
R. Banerjee, H. Furukawa, D. Britt, C. Knobler, M. O’Keeffe, O.M. Yaghi, Control of Pore Size and Functionality in Isoreticular Zeolitic Imidazolate Frameworks and their Carbon Dioxide Selective Capture Properties. J. Am. Chem. Soc. 131, 3875–3877 (2009)
Y. Liu, E. Hu, E.A. Khan, Z. Lai, Synthesis and characterization of zif-69 membranes and separation for co2/co mixture. J. Membr. Sci. 353, 36–40 (2010)
A. Battisti, S. Taioli, G. Garberoglio, Zeolitic imidazolate frameworks for separation of binary mixtures of co2, ch4, n2 and h2: A computer simulation investigation. Microporous Mesoporous Mater. 143, 46–53 (2011)
T. Chokbunpiam, S. Fritzsche, C. Chmelik, J. Caro, W. Janke, S. Hannongbua, Gate opening, diffusion, and adsorption of co2 and n2 mixtures in zif-8. J. Phys. Chem. C 120, 23458–23468 (2016)
M. Zeeshan, V. Nozari, M.B. Yagci, T. Isık, U. Unal, V. Ortalan, S. Keskin, A. Uzun, Core-shell type ionic liquid/metal organic framework composite: An exceptionally high co2/ch4 selectivity. J. Am. Chem. Soc. 140, 10113–10116 (2018)
B. Wang, A.P. Cote, H. Furukawa, M. O’Keeffe, O.M. Yaghi, Colossal cages in zeolitic imidazolate frameworks as selective carbon dioxide reservoirs. Nature 453, 207–U6 (2008)
Q. Wang, H. Wang, S. Peng, X. Peng, D. Cao, Adsorption and separation of xe in metal-organic frameworks and covalent-organic materials. J. Phys. Chem. C 118, 10221–10229 (2014)
O.V. Magdysyuk, F. Adams, H.-P. Liermann, I. Spanopoulos, P.N. Trikalitis, M. Hirscher, R.E. Morris, M.J. Duncan, L.J. McCormick, R.E. Dinnebier, Understanding the adsorption mechanism of noble gases kr and xe in cpo-27-ni, cpo-27-mg, and zif-8. Phys. Chem. Chem. Phys. 16, 23908–23914 (2014)
T. Wu, J. Lucero, M.A. Sinnwell, P.K. Thallapally, M.A. Carreon, Recovery of xenon from air over zif-8 membranes. Chem. Commun. 54, 8976–8979 (2018)
T. Wu, J. Lucero, Z. Zong, S.K. Elsaidi, P.K. Thallapally, M.A. Carreon, Microporous crystalline membranes for kr/xe separation: comparison between alpo-18, sapo-34, and zif-8. ACS Appl. Nano Mater. 1, 463–470 (2018)
Y. Gurdal, S. Keskin, Atomically detailed modeling of metal organic frameworks for adsorption, diffusion, and separation of noble gas mixtures. Ind. Eng. Chem. Res. 51, 7373–7382 (2012)
Y. Gurdal, S. Keskin, Predicting noble gas separation performance of metal organic frameworks using theoretical correlations. J. Phys. Chem. C 117, 5229–5241 (2013)
Y. Gürdal Durğun. Computational assessment of zeolitic-imidazolate frameworks (zifs) for adsorption and diffusion based separation of noble gas mixtures. Bitlis Eren Üniversitesi Fen Bilimleri Dergisi 2019, 8, 1009–1018
Y. Gurdal, S. Keskin. A new approach for predicting gas separation performances of mof membranes. J. Membr. Sci. 20160, 519, 45–54
M.J. Sanborn, R.Q. Snurr, Diffusion of binary mixtures of cf4 and n-alkanes in faujasite. Sep. Purif. Technol. 20, 1–13 (2000)
R. Krishna, J.M. van Baten, In silico screening of zeolite membranes for co2 capture. J. Membr. Sci. 360, 323–333 (2010)
S. Keskin, D.S. Sholl, Efficient methods for screening of metal organic framework membranes for gas separations using atomically detailed models. Langmuir 25, 11786–11795 (2009)
E.-Y. Chen, Y.-C. Liu, M. Zhou, L. Zhang, Q. Wang, Effects of structure on hydrogen adsorption in zeolitic imidazolate frameworks. Chem. Eng. Sci. 71, 178–184 (2012)
E.L. First, C.A. Floudas, Mofomics: Computational pore characterization of metal-organic frameworks. Microporous Mesoporous Mater. 165, 32–39 (2013)
D. Wu, C. Wang, B. Liu, D. Liu, Q. Yang, C. Zhong, Large-scale computational screening of metal-organic frameworks for ch4/h2 separation. AIChE J. 58, 2078–2084 (2012)
J.C. Tan, T.D. Bennett, A.K. Cheetham, Chemical structure, network topology, and porosity effects on the mechanical properties of zeolitic imidazolate frameworks. Proc. Nat. Acad. Sci. U.S.A. 107, 9938–9943 (2010)
E. Gulcay, I. Erucar, Molecular simulations of cofs, irmofs and zifs for adsorption-based separation of carbon tetrachloride from air. J. Mol. Graph. Model. 86, 84–94 (2019)
J. Pérez-Pellitero, H. Amrouche, F. Siperstein, G. Pirngruber, C. Nieto-Draghi, G. Chaplais, A. Simon-Masseron, D. Bazer-Bachi, D. Peralta, N. Bats, Adsorption of co2, ch4, and n2 on zeolitic imidazolate frameworks: Experiments and simulations. Chemistry A 16, 1560–1571 (2010)
L. Verlet. Computer “experiments” on classical fluids. i. thermodynamical properties of lennard-jones molecules. Phys. Rev. 1967, 159, 98–103
A.K. Rappe, C.J. Casewit, K.S. Colwell, W.A. Goddard, W.M. Skiff, Uff, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 114, 10024–10035 (1992)
E. Atci, S. Keskin, Understanding the potential of zeolite imidazolate framework membranes in gas separations using atomically detailed calculations. J. Phys. Chem. C 116, 15525–15537 (2012)
T. Van Heest, S.L. Teich-McGoldrick, J.A. Greathouse, M.D. Allendorf, D.S. Sholl, Identification of metal-organic framework materials for adsorption separation of rare gases: Applicability of ideal adsorbed solution theory (iast) and effects of inaccessible framework regions. J. Phys. Chem. C 116, 13183–13195 (2012)
G. Boato, G. Casanova, A self-consistent set of molecular parameters for neon, argon, krypton and xenon. Physica 27, 571–589 (1961)
D. Frenkel, B. Smit, Understanding Molecular Simulation: From Algorithms to Applications (Academic Press, San Diego, 2002)
D.C. Rapaport, The Art of Molecular Dynamics Simulation (Cambridge University Press, Cambridge, 2004)
D.J. Evans, B.L. Holian, The nose-hoover thermostat. J. Chem. Phys. 83, 4069–4074 (1985)
S.-J. Lee, T.-U. Yoon, A.-R. Kim, S.-Y. Kim, K.-H. Cho, Y.K. Hwang, J.-W. Yeon, Y.-S. Bae, Adsorptive separation of xenon/krypton mixtures using a zirconium-based metal-organic framework with high hydrothermal and radioactive stabilities. J. Hazard. Mater. 320, 513–520 (2016)
C.A. Fernandez, J. Liu, P.K. Thallapally, D.M. Strachan, Switching kr/xe selectivity with temperature in a metal-organic framework. J. Am. Chem. Soc. 134, 9046–9049 (2012)
L. Li, L. Guo, Z. Zhang, Q. Yang, Y. Yang, Z. Bao, Q. Ren, J. Li, A robust squarate-based metal-organic framework demonstrates record-high affinity and selectivity for xenon over krypton. J. Am. Chem. Soc. 141, 9358–9364 (2019)
M.V. Parkes, C.L. Staiger, J.J. Perry IV, M.D. Allendorf, J.A. Greathouse, Screening metal-organic frameworks for selective noble gas adsorption in air: effect of pore size and framework topology. Phys. Chem. Chem. Phys. 15, 9093–9106 (2013)
R. Anderson, B. Schweitzer, T. Wu, M.A. Carreon, D.A. Gómez-Gualdrón, Molecular simulation insights on xe/kr separation in a set of nanoporous crystalline membranes. ACS Appl. Mater. Interfaces 10, 582–592 (2018)
T. Wu, X. Feng, S.K. Elsaidi, P.K. Thallapally, M.A. Carreon, Zeolitic imidazolate framework-8 (zif-8) membranes for kr/xe separation. Ind. Eng. Chem. Res. 56, 1682–1686 (2017)
D. Fairen-Jimenez, S.A. Moggach, M.T. Wharmby, P.A. Wright, S. Parsons, T. Düren, Opening the gate: Framework flexibility in zif-8 explored by experiments and simulations. J. Am. Chem. Soc. 133, 8900–8902 (2011)
D. Peralta, G. Chaplais, A. Simon-Masseron, K. Barthelet, C. Chizallet, A.-A. Quoineaud, G.D. Pirngruber, Comparison of the behavior of metal-organic frameworks and zeolites for hydrocarbon separations. J. Am. Chem. Soc. 134, 8115–8126 (2012)
L. Diestel, H. Bux, D. Wachsmuth, J. Caro, Pervaporation studies of n-hexane, benzene, mesitylene and their mixtures on zeolitic imidazolate framework-8 membranes. Microporous Mesoporous Mater. 164, 288–293 (2012)
Acknowledgements
The numerical calculations reported in this paper were partially performed at TUBITAK ULAKBIM, High Performance and Grid Computing Center (TRUBA resources), located in Turkey. This work is financially supported by Adana Alparslan Turkes Science and Technology University Scientific Research Project Office under Project ID: 19103009.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that she has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gurdal, Y. Theoretical investigation of ZIFs as adsorbents or membranes for separating noble gas mixtures: applying a newer method for predicting performances. J Porous Mater 28, 905–917 (2021). https://doi.org/10.1007/s10934-021-01045-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-021-01045-9