Abstract
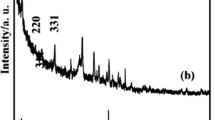
Zeolites with substitution by transition metals are expected to have unique catalytic properties in addition to common cation exchange abilities, but studies on the synthesis of iron-substituted zeolites with a greater cation exchange capacity (CEC) are very few. We hydrothermally synthesized iron-substituted Na-P1 type zeolites having CEC values of >300 cmolc kg−1 with iron content of up to 90 cmolc kg−1 with changing the addition of iron. Most of the iron in the products was concluded to be incorporated into the structure of Na-P1 by substituting aluminum, because measured CEC value and the content of sodium (exchangeable cation) nearly coincided with the sum of aluminum and iron contents in each product. In addition, UV–Visible diffuse reflectance spectra of the products revealed characteristic bands of isolated tetrahedral iron species and Fourier Transform Infrared spectroscopy (FT-IR) results indicated the existence of Si-O-Fe bonds in the products. These results confirmed the substitution of iron in the framework of Na-P1 by a hydrothermal synthesis in a short time.






Similar content being viewed by others
References
M.E. Davis, Microporous Mesoporous. Mater. 21, 173 (1998)
J. Weitkamp, Solid State Ionics 131, 175 (2000)
S.E. Bailey, T.J. Olin, R.M. Bricka, D.D. Adrian, Water Res. 33(11), 2469 (1999)
E. Erdem, N. Karapinar, R. Donat, J. Colloid Interf. Sci. 280, 309 (2004)
L.G.A. van de Water, J.C. van der Waal, J.C. Jansen, M. Cadoni, L. Marchese, T. Maschmeyer, J. Phys. Chem. B 107, 10423 (2003)
R. Fricke, H. Kosslick, G. Lischke, M. Richter, Chem. Rev. 100, 2303 (2000)
P. Ratnasamy, R. Kumar, Catal. Today 9(4), 329 (1991)
M. Tamura, W. Chaikittisilp, T. Yokoi, T. Okubo, Microporous Mesoporous. Mater. 112, 202 (2008)
G. Centi, S. Perathoner, F. Trifiró, A. Aboukais, C.F. Aïssi, M. Guelton, J. Phys. Chem-US 96, 2617 (1992)
K. Na, C. Jo, J. Kim, W.S. Ahn, R. Ryoo, ACS Catal. 1, 901 (2011)
K. Chalupka, C. Thomas, Y. Millot, F. Averseng, S. Dzwigaj, J. Catal. 305, 46 (2013)
J.H. Yun, R.F. Lobo, J. Catal. 312, 263 (2014)
A. Ribera, I.W.C.E. Arends, S. de Vries, J. Pérez-Ramírez, R.A. Sheldon, J. Catal. 195, 287 (2000)
E.J.M. Hensen, Q. Zhu, R.A.J. Janssen, P.C.M.M. Magusin, P.J. Kooyman, R.A. van Santen, J. Catal. 233, 123 (2005)
R. Szostak, T.L. Thomas, J. Chem. Soc., Chem. Commun. 2, 113 (1986)
K. Katsuki, S. Yoneoka, N. Mori, M. Hasegawa, Y. Yamamoto, Y. Yoshino, J. Porous Mater. 15, 35 (2008)
C.V.A. Duke, K. Latham, C.D. Williams, Zeolites 15, 213 (1995)
P. Ratnasamy, A.N. Kotasthane, V.P. Shiralkar, A. Thangaraj, S. Ganapathy, in ACS Symposium Series 398, ed. by M.L. Occelli, H.E. Robson (American Chemical Society, Washington, D.C., 1989), p. 405
R. Kumar, A. Raj, S.B. Kumar, P. Ratnasamy, Stud. Surf. Sci. Catal. 84, 109 (1994)
S. Hansen, Acta Crystallogr. C 46, 1361 (1990)
U. Håkansson, L. Fälth, Acta Crystallogr. C 46, 1363 (1990)
B.R. Albert, A.K. Cheetham, J.A. Stuart, C.J. Adams, Microporous Mesoporous. Mater. 21, 133 (1998)
P. Sharma, J.-S. Song, M.H. Han, C.H. Cho, Sci. Rep. (2016). doi:10.1038/srep22734
M. Maldonado, M.D. Oleksiak, S. Chinta, J.D. Rimer, J. Am. Chem. Soc. 135, 2641 (2013)
M.L. Jackson, Soil Chemical Analysis Advanced Course (University of Wisconsin, Madison, 1956), pp. 47–58
K. Katsuki, M. Okamoto, E. Ichikawa, A. Iwashina, S. Koike, Y. Yamamoto, T. Takeuchi, Y. Yoshino, Nippon Kagaku Kaishi 9, 689 (1995) (Japanese)
Ch. Baerlocher, W.M. Meier, Z. Kristallogr. Cryst. Mater. 135, 339 (1972)
Y.S. Ko, W.S. Ahn, Microporous Mater., 9, 131 (1997)
S. Shevade, R.K. Ahedi, A.N. Kotasthane, Catal. Lett. 49, 69 (1997)
P. Wu, T. Komatsu, T. Yashima, Microporous Mesoporous. Mater. 20, 139 (1998)
D. Goldfarb, M. Bernardo, K.G. Strohmaier, D.E.W. Vaughan, H. Thomann, J. Am. Chem. Soc. 116, 6344 (1994)
S. Bordiga, R. Buzzoni, F. Geobaldo, C. Lamberti, E. Giamello, A. Zecchina, G. Leofanti, G. Petrini, G. Tozzola, G. Vlaic, J. Catal. 158, 486 (1996)
J. Pérez-Ramírez, J.C. Groen, A. Brückner, M.S. Kumar, U. Bentrup, M.N. Debbagh, L.A. Villaescusa, J. Catal. 232, 318 (2005)
U. Schwertmann, R.M. Cornell, Iron Oxides in the Laboratory (Wiley-VCH, Weinheim, 2000), pp. 67–134
E.M. Flanigen, H. Khatami, H.A. Szymanski, in Advance in Chemistry Series 101, ed. by E.M. Flanigen, L.B. Sand (American Chemical Society, Washington, D.C., 1971), p. 201
R. Szostak, V. Nair, T.L. Thomas, J. Chem. Soc., Faraday Trans. 1 83, 487 (1987)
P. Castaldi, L. Santona, C. Cozza, V. Giuliano, C. Abbruzzese, V. Nastro, P. Melis, J. Mol. Struct. 734, 99 (2005)
M. Salavati-Niasari, J. Incl. Phenom. Macrocycl. 65, 317 (2009) doi:10.1007/s10847-009-9585-y
A. Nezamzadeh-Ejhieh, S. Hushmandrad Appl. Catal. A 388, 149 (2010)
Acknowledgements
We thank Associate Professor Dr. Satoshi Mitsunobu for providing of the iron oxides and Mr. Takeshi Kiyoi at the Division of Analytical Bio-medicine the Advanced Research Support Center, Ehime University for his technical assistance of SEM observation. Additionally this study was supported by Ehime Institute of Industrial Technology for using UV–Visible spectroscopy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumon, A., Abidin, Z. & Matsue, N. Synthesis of iron substituted zeolite with Na-P1 framework. J Porous Mater 24, 1061–1068 (2017). https://doi.org/10.1007/s10934-016-0346-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-016-0346-1