Abstract
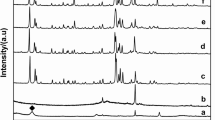
In this research, hydrothermal/alkali fusion synthesis of zeolite NaX and NaA was reported by using pyrophyllite as the source of Si and Al. Characterization of synthesized compounds was carried out by X-ray diffraction, X-ray fluorescence, FESEM (field emission scanning electron microscopy), and BET (Brunauer–Emmett–Teller) analyses. To activate pyrophyllite, two methods including calcination and alkali fusion were employed. Calcination of pyrophyllite resulted in the dehydroxylation process. Four batch formulas were set to the synthesis of zeolite structure from dehydroxylated pyrophyllite which resulted in producing sodalite zeolite. Results showed that alkali fusion is an effective method for the activation of pyrophyllite. Zeolite NaA with a crystallinity of about 56% was successfully synthesized at 90 °C with an average particle size around 0.085 μm. In the case of zeolite NaX, the synthesis was performed at 120 °C while the particle size is around 1.73 μm. However, it was found that for producing a high crystallinity of zeolite NaX, longer crystallization time is needed. BET results revealed that the specific surface area of the synthesized zeolite NaX is around 12 m2/gr with an average pore volume of 0.054 cm3/g.







Similar content being viewed by others
References
Ahadi A, Rostamnia S, Panahi P, Wilson L D, Kong Q, An Z, and Shokouhimehr M, Catalysts 9 (2019) 140.
Kim A, Rafiaei S M, Abdolhosseini S, and Shokouhimehr M, Energy Environ Focus 4 (2015) 18.
Nasrollahzadeh M, Baran T, Baran N Y, Sajjadi M, Tahsili M, and Shokouhimehr M, Sep Purif Technol 239 (2020) 116542.
Rafiaei S M, Ceram Int 48 (2022) 14913.
Habibi M K, Rafiaei S M, Alhaji A, and Zare M, J Mol Struct 1228 (2021) 129769.
Habibi M K, Rafiaei S M, Alhaji A, and Zare M, J Alloys Compd 890 (2021) 161901.
Khaleque A, Alam M M, Hoque M, Mondal S, Haider J B, Xu B, Johir M, Karmakar A K, Zhou J, and Ahmed M B, Environ Adv 2 (2020) 100019.
Sajjadi M, Nasrollahzadeh M, Ghafuri H, Baran T, Orooji Y, Baran N Y, and Shokouhimehr M, Int J Biol Macromol 209 (2022) 1573.
Salmankhani A, Mousavi Khadem S S, Seidi F, Mashhadzadeh A H, Zarrintaj P, Habibzadeh S, Mohaddespour A, Rabiee N, Lima E C, Shokouhimehr M, Varma R S, and Saeb M, Chem Pap 75 (2021) 6217.
Moshoeshoe M, Nadiye-Tabbiruka M S, and Obuseng V, Am J Mater Sci 7 (2017) 196.
Kulprathipanja S, Zeolites in Industrial Separation and Catalysis, John Wiley & Sons (2010).
Setthaya N, Chindaprasirt P, and Pimraksa K, Preparation of zeolite nanocrystals via hydrothermal and solvothermal synthesis using of rice husk ash and metakaolin, p 242
Tsujiguchi M, Kobashi T, Oki M, Utsumi Y, Kakimori N, and Nakahira A, J Asian Ceram Soc 2 (2014) 27.
Sapawe N, Jalil A, Triwahyono S, Shah M, Jusoh R, Salleh N, Hameed B, and Karim A, Chem Eng J 229 (2013) 388.
El-Naggar M, El-Kamash A, El-Dessouky M, and Ghonaim A, J Hazard Mater 154 (2008) 963.
Gaidoumi A E, Benabdallah A C, Bali B E, and Kherbeche A, Arab J Sci Eng 43 (2018) 191.
Qiang Z, Shen X, Guo M, Cheng F, and Zhang M, Microporous Mesoporous Mater 287 (2019) 77–84.
Garshasbi V, Jahangiri M, and Anbia M, Appl Surf Sci 393 (2017) 225.
Kim W, Chae W, Kwon S, Kim K, Lee H, and Kim S, Mater Trans 55 (2014) 1488.
Li G, Zeng J, Luo J, Liu M, Jiang T, and Qiu G, Appl Clay Sci 99 (2014) 282.
Ríos C A, Williams C D, and Castellanos O M, Ing Compet 14 (2012) 125.
Chandrasekhar S, and Pramada P, J Porous Mater 6 (1999) 283.
Khalifa A Z, Cizer Ö, Pontikes Y, Heath A, Patureau P, Bernal S A, and Marsh A T, Cem Concr Res 132 (2020) 106050.
Zhang Z, and Wang L, J Chin Ceram Soc 26 (1998) 618.
Johnson E B G, and Arshad S E, Appl Clay Sci 97–98 (2014) 215.
Zhang X, Tang D, Zhang M, and Yang R, Powder Technol 235 (2013) 322.
Ma Y, Yan C, Alshameri A, Qiu X, and Zhou C, Adv Powder Technol 25 (2014) 495.
Ghodrati M, Rafiaei S M, and Tayebi L, J Mech Behav Biomed Mater 145 (2023) 106001.
Schomburg J, Thermochim Acta 93 (1985) 521.
Abdullahi T, Harun Z, and Othman M H D, Adv Powder Technol 28 (2017) 1827.
Sanchez-Soto P J, and Perez-Rodriguez J L, Thermochim Acta 138 (1989) 267.
Chen Z, Qing H, Zhou K, Sun D, and Wu R, Prog Mater Sci 108 (2020) 100618.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Habibi, M.K., Rafiaei, S.M. Hydrothermal/Alkali Fusion Synthesis of Zeolite NaA and NaX from Pyrophyllite Mineral Clay. Trans Indian Inst Met (2024). https://doi.org/10.1007/s12666-024-03272-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12666-024-03272-5