Abstract
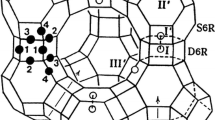
The single-crystal structure of |Zn35.5|[Si121Al71O384]-FAU per unit cell, a = 24.794(1), dehydrated at 673 K and 1 × 10−6 Torr, has been determined by single-crystal X-ray diffraction techniques in the space group \( Fd\bar{3}m \) at 294(1) K. The structure was refined using all intensities to the final error indices (using the 930 reflections for which F o > 4σ(F o)) R 1 = 0.0448 (based on F) and wR 2 = 0.1545 (based on F 2). About 35.5 Zn2+ ions per unit cell are found at an unusually large number of crystallographic distinct positions, six. The 0.5 Zn2+ ion per unit cell is located at the center of double 6-ring (D6R, site I; Zn(I)-O(3) = 2.642(3) Å and O(3)-Zn(I)-O(3) = 81.23(12) and 98.77(12)°). Two different site-I′ positions (in the sodalite cavities opposite D6Rs) are occupied by 14 and 3 Zn2+ ions per unit cell, respectively; these Zn2+ ions are recessed 0.67 Å and 1.02 Å, respectively, into the sodalite cavities from their 3-oxygens plane (Zn(I′a)-O(3) = 2.094(3) Å, Zn(I′b)-O(3) = 2.23(5) Å, O(3)-Zn(I′a)-O(3) = 110.32(12)°, and O(3)-Zn(I′b)-O(3) = 100.9(30)°). Site-II′ positions (in the sodalite cavities opposite S6Rs) are occupied by 6 Zn2+ ions, each of which extends 0.63 Å into the sodalite cavities from their 3-oxygens plane (Zn(II′)-O(2) = 2.164(3) Å and O(2)-Zn(II′)-O(2) = 112.00(12)°). Twelve Zn2+ ions are found at two nonequivalent sites II (in the supercage) with occupancies of 7 and 5 ions, respectively; these Zn2+ ions are recessed 0.52 Å and 0.96 Å, respectively, into the supercage from their 3-oxygens plane (Zn(IIa)-O(2) = 2.138(12) Å, Zn(IIb)-O(2) = 2.28(4) Å, O(2)-Zn(IIa)-O(2) = 114.2(10)°, and O(2)-Zn(IIb)-O(2) = 103.7(25)°).





Similar content being viewed by others
References
K.H. Rhee, F.R. Brown, D.H. Finseth, J.M. Stencel, Zeolites 3, 344 (1983)
P. Chu, US Patent No. 4120910 (1978)
Kh.M. Minachev, D.A. Kondrat’ev, Usp. Khim. 52, 1921 (1983)
Kh.M. Minachev, D.A. Kondrat’ev, A.A. Dergachev, et al. Izv. Akad. Nauk SSSR, Ser. Khim. 1304 (1981)
T.M. Gairbekov, M.I. Takaeva, A.K. Manovyan, Chem. Technol. Fuels Oils 25, 473 (1989)
Kh.M. Minachev, A.A. Derachev, M.S. Kharson, Dokl. Phys. Chem. 300, 427 (1988)
N. Kumar, L.E. Lindfors, R. Byggningsbacka, Appl. Catal. 139, 189 (1996)
T.F. Brownscombe, US Patent No. 5053372, (1991)
M. Ziolek, K. Nowinska, K. Lecksowska, Zeolites 12, 710 (1992)
C.J. Blower, T.D. Smith, Zeolites 13, 394 (1993)
C. Brooks, Sep. Sci. Technol. 25, 1817 (1990)
A. Maes, A. Cremers, J. Chem. Soc. Faraday Trans. 71, 265 (1995)
Y.H. Yeom, Y. Kim, K. Seff, J. Phys. Chem. B 101, 5314 (1997)
D. Bae, S. Zhen, K. Seff, J. Phys. Chem. B 103, 5631 (1999)
M.F. Ciraolo, P. Noby, J.C. Hanson, D.R. Corbin, C.P. Grey, J. Phys. Chem. B 103, 346 (1999)
S.H. Lee, Y. Kim, Bull. Korean Chem. Soc. 21, 180 (2000)
D. Bae, K. Seff, Micropor. Mesopor. Mater. 40, 233 (2000)
H. Kachun, Zeolites 15, 377 (1995)
L.B. McCusker, K. Seff, J. Phys. Chem. 85, 405 (1981)
S.M. Seo, G.H. Kim, H.S. Lee, S.O. Ko, O.S. Lee, Y.H. Kim, S.H. Kim, N.H. Heo, W.T. Lim, Anal. Sci. 22, x209 (2006)
W.T. Lim, S.M. Seo, G.H. Kim, H.S. Lee, K. Seff, J. Phys. Chem. C 111, 18294 (2007)
Z. Otwinowski, W. Minor, Methods Enzymol. 276, 307 (1997)
Bruker-AXS (ver. 6.12), XPREP, Program for the Automatic Space Group Determination (Bruker AXS Inc., Madison, Wisconsin, USA, 2001)
G.M. Shwldrick, SHELXL97, Program for the Refinement of Crystal Structures (University of Gottingen, Germany, 1997)
W.T. Lim, S.Y. Choi, J.H. Choi, Y.H. Kim, N.H. Heo, S.H. Kim, K. Seff, Micropor. Mesopor. Mater. 92, 234 (2006)
P.A. Doyle, P.S. Turner, Acta Crystallogr. A 24, 390 (1968)
J.A. Ibers, W.C. Hamilton, in International Table for X-ray Crystallography, vol. IV, ed. by J.A. Ibers, W.C. Hamilton (Kynoch Press, Birmingham, England, 1974), pp. 71–98
D.T. Cromer, Acta Crystallogr. 18, 17–23 (1965)
J.A. Ibers, W.C. Hamilton, International Table for X-ray Crystallography, vol IV (Kynoch Press, Birmingham, England, 1974), pp. 148–150
W. Loewenstein, Am. Mineral. 39, 92 (1954)
J.V. Smithm, Molecular Sieve Zeolites-I, in Advances in Chemistry Series, vol. 101 ed. by E.M. Flanigen, L.B. Sand (American Chemical Society, Washinton, DC, 1971), pp. 171–200
Y.H. Yeom, S.B. Jang, S.H. Song, Y. Kim, K. Seff, J. Phys. Chem. B 101, 6914 (1997)
M.J. Kim, M.S. Jeong, Y. Kim, K. Seff, Micro. Meso. Mater. 30, 233 (1999)
S.M. Seo, S.Y. Choi, J.M. Seo, K.J. Jung, N.H. Heo, W.T. Lim, Bull. Korean Chem. Soc. 30(8), 1703 (2009)
Y.H. Yeom, A.N. Kim, Y. Kim, S.H. Song, K. Seff, J. Phys. Chem. B 102, 6071 (1998)
E.Y. Choi, Y. Kim, K. Seff, J. Phys. Chem. B 106, 5827 (2002)
G.H. Jeong, Y. Kim, K. Seff, Langumuir 20, 9354 (2004)
Acknowledgments
The authors thank the staff at beamline 4A MXW of Pohang Light Source, Korea, for assistance during data collection. This study was carried out with the support of Cooperative Research Program for Agricultural Science & Technology Development (200901OFT102966074), RDA, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Seo, S.M., Kim, H.S., Park, M. et al. Synthesis and structural refinement of fully dehydrated fully Zn2+-exchanged zeolite Y (FAU), |Zn35.5|[Si121Al71O384]-FAU. J Porous Mater 18, 47–56 (2011). https://doi.org/10.1007/s10934-010-9355-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-010-9355-7