Abstract
Preventing biodiversity loss is a key aim of modern conservation, and paleolimnology can inform conservation strategies for target species and habitats where other data are unavailable. Care must be taken to fully understand the possibilities and limits of such techniques, particularly where they concern single species. This study uses plant and seed distribution data to inform macrofossil reconstructions of the rare macrophyte Najas flexilis (Slender Naiad) in Scotland, UK. It answers three questions: (a) How does the location of N. flexilis seeds in the surface sediments relate to the distribution of N. flexilis plants? (b) How do the numbers of seeds in surface sediments correlate with % cover of N. flexilis plants across lakes with differing N. flexilis abundances? (c) What are the implications of these findings for paleolimnology? Percentage N. flexilis cover and number of N. flexilis seeds in surface sediments were recorded at ~100 sample points at each of three sites; one where the species was abundant, one where it was occasional and one where it was extinct. At all sites, N. flexilis seeds were present in surface sediments across the entire lake. No correlation between % cover N. flexilis and the number of seeds in surface sediments was found within individual sites. The distribution of seeds in these lakes appeared to be related to multiple environmental and ecological variables including latitude and longitude (proxies for water currents). This is attributed to the ability of seed-bearing N. flexilis plants to fragment and float large distances on water. Between sites, there was a significant difference in the mean seed counts, with higher mean seed counts corresponding to higher abundances of N. flexilis plants. It is concluded that N. flexilis is likely to be well represented in sediment cores taken from any location within a basin, but that care should be taken when inferring changes in N. flexilis abundance from changes in the numbers of seeds in sediment samples. This work demonstrates that the reproductive ecology (number of seeds produced and dispersal mechanisms) is an important factor to consider when attempting reconstructions of single aquatic plant populations from macrofossil records.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, freshwater ecosystems are suffering declines in biodiversity at far greater rates than terrestrial ecosystems due to overexploitation, water pollution, flow modification, habitat destruction and degradation, and invasion by exotic species (Dudgeon et al. 2006). Preventing biodiversity loss is a central concern of modern conservation, and the preservation of “rare” or “uncommon” species is a key aspect of this approach (Loreau et al. 2001). However, long term records of the changing presence and abundance of such species through time are often lacking (Rondinini et al. 2006). To carry out effective, evidence-based conservation, this data gap must be urgently addressed (Sutherland et al. 2004). Paleolimnology is traditionally used as a tool to extend short term records of environmental variables into the past, and typically uses information on changing species composition as a proxy for water quality, yet the potential to reconstruct single aquatic plant populations from sediment records has not been fully explored.
Ecological theory states that the presence of a plant at any location depends upon the ability of the species to (a) disperse and (b) colonise that site (MacArthur and Wilson 1967). The distribution of the reproductive parts of plants that are laid down in the sediment and later analysed by paleolimnologists as macrofossils represent the dispersal stage of this process. The relationship between distribution of seeds in the sediments and plants within the lake is an important, but often overlooked, criteria for the interpretation of macrofossil reconstructions. Birks (1973) critically analysed this relationship for all wetland and aquatic plants found in 32 lakes in Minnesota. For all plants, the distribution of seed macrofossils was related to the dispersal strategy of the plant—for example, seed macrofossils of Ranunculus sceleratus, whose ovules float on water, were found predominantly in areas of open water. In general, macrofossils of obligate aquatic plants were only found in small areas located close to parent plants; this was attributed to the fact that such species produce heavy seeds with no dispersal mechanism. Macrofossils therefore typically represent only local vegetation changes, and macrofossil assemblages from different parts of the same lake may differ (Birks 2006). These patterns were echoed in macrofossil distribution studies in the UK (Zhao et al. 2006) and Turkey (Levi et al. 2014). Despite this, macrofossil reconstructions based upon a single core are common, and rely upon the assumption that spatial distribution of macrofossils is even across a lake basin (Birks 1995; Birks 2006). Strategies for choosing core location are often based upon (a) proximity to the littoral zone; (b) ease of sampling and access; and (c) known/assumed areas of sediment accumulation, rather than present day plant distribution (Birks 2006).
In Minnesota, Birks (1973) noted three exceptions to the general, localised representation of obligate aquatic plants as seed macrofossils; Chara spp, Nitella opaca agg., and Najas flexilis. The oospores of the Characeae are small and easily distributed by water currents, and this has been observed in surface sediments and in sediment cores across the world (Zhao et al. 2006; Madgwick et al. 2010; Levi et al. 2014). However, no such dispersal mechanism is present in N. flexilis, which produces relatively large, heavy seeds. In the UK, N. flexilis draws protected species status as a ‘Priority Species’ in the European Habitats Directive (1992) on the basis that, although quantitative data are limited, disappearance of the plant from its two former English localities (Lake Windermere and Esthwaite Water) suggests that it is threatened. In Poland, declines and disappearances of N. flexilis seeds in cores from two lowland forest lakes appear to correlate with changes in trophic status, with N. flexilis decline correlating with an increase in Sphagnum spp. remains in one core (attributed to nutrient depletion/acidification) and an increase in Botryococcus spp. in the other core (attributed to eutrophication) (Gałka et al. 2012). In contrast, the paleolimnological work of Bennion et al. (2008, 2010), which included seven UK lakes currently or formerly supporting N. flexilis, did not support the theory that N. flexilis is in decline. Instead, it appeared that some sites may have been too unproductive for N. flexilis in the past, with macrofossil remains of the species appearing alongside diatom and cladocera assemblages indicative of mild eutrophication. However, in both of these studies inferences of the abundance of N. flexilis were based upon single cores with no consideration given to the extent to which they represent the site as a whole. It is clear that more detailed paleolimnological work is required to further investigate the apparent decline of N. flexilis. To facilitate this, the representation of the plant in the sediment of UK lakes as plant macrofossils must be better understood.
This paper explores the ways in which knowledge of seed dispersal patterns might inform paleolimnological macrofossil reconstructions of N. flexilis. The key questions are:
-
a.
How do distributions of N. flexilis plants relate to distributions of N. flexilis seeds within surface sediments?
-
b.
How do N. flexilis seed abundances in surface sediments relate to N. flexilis cover across lakes with differing abundances of N. flexilis?
-
c.
What implications do these findings have for coring strategies and the interpretation of macrofossil records when reconstructing N. flexilis using paleolimnology?
The extent to which temporal changes in N. flexilis presence and abundance can be represented in sediment cores will be investigated by examining the contemporary spatial distributions of N. flexilis plants and seeds at three lakes: one lake at which N. flexilis is abundant, one at which it is occasional and one at which it is extinct.
Study sites
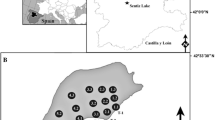
Three study sites were selected from a database containing all of the existing N. flexilis records for UK lakes over the last 150 years: Upper Glenastle Loch (latitude 55.621389; longitude −6.290278), Tangy Loch (latitude 55.492213; longitude −5.652543) and Loch of Butterstone (latitude 56.585862; longitude −3.533379) (see Fig. 1). Together, these sites represent lakes with N. flexilis distributions from highly abundant (Upper Glenastle Loch) and moderately abundant (Tangy Loch) to recently extinct (Loch of Butterstone).
Upper Glenastle Loch is the first in a chain of two lochs on the 4km long Glen Golach, a river located on the Oa peninsula on the island of Islay in the Scottish Hebrides. The loch is 7 ha in area, with a small catchment of ~250 ha. It is a shallow (1.5 m average depth) lake with a westerly prevailing wind (RenSmart 2016). Past surveys show that N. flexilis was present in Upper Glenastle Loch in 1994, 1998, 1999 and 2010, however none of these records indicate the extent or distribution of N. flexilis cover.
Tangy Loch is located on the Kintyre peninsula. It is a small (18 ha), shallow (average depth 1.3 m) lake with a 175 ha catchment and a prevailing wind from west-south-west (RenSmart 2016). Past surveys found N. flexilis in Tangy loch in 1973, 1977, 1978, 1983, 1989, 1994, 1998 and 2009 and 2014. The most recent survey indicated a small but healthy population of the plant. (National Biodiversity Network 2014; Scottish Natural Heritage, pers. commun., Walmisley, pers. commun.).
Loch of Butterstone is the third lake in a chain of five along the river Lunan Burn in Perthshire, central Scotland. It has an average depth of 3.5 m, an area of 43 ha and a prevailing wind from west-south-west (RenSmart 2016). The catchment is much larger than that of Upper Glenastle Loch, at about 1800 ha. Past surveys show that N. flexilis was present in Loch of Butterstone in 1986, 1994, 1996, 1997, 1999 and 2004 (James and Barclay 1996, unpublished; Wingfield, 2006). Surveys in 2006 and 2010 did not find the plant, consequently N. flexilis is believed to be extinct at the site.
Materials and Methods
The ecology of N. flexilis
N. flexilis prefers sites with a circumneutral pH and alkalinities between 6 mg l−1 and 308 mg l−1 (Moyle 1945). In the UK, most sites containing N. flexilis are located on base rich mineral deposits but have upland, acidic catchments, which act as a buffer to high pH levels (Wingfield et al. 2004). It grows in relatively deep water, and has been reported growing at depths of up to 14.0 m in Shoal Lake, Ontario, Canada (Pip and Simmons 1986). It is commonly found at depths of 1.5–3 m in the UK (Preston and Croft 1997). N. flexilis is an annual, and, unusually for an aquatic plant, is unable to reproduce vegetatively (Hutchinson 1975). It therefore relies upon its large, elliptical seeds to disperse. Because it does not overwinter, N. flexilis has a short growing season—typically from May to October in the UK (Wingfield 2004). The sites and methods used in this study were chosen to best represent typical N. flexilis habitats and maximum annual N. flexilis distributions.
Methods
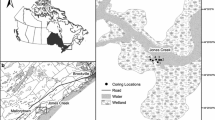
Macrophyte distribution and surface sediment surveys were carried out at each of the three lochs in August, when N. flexilis is typically most abundant (Upper Glenastle Loch and Loch of Butterstone in 2013 and Tangy Loch in 2014). Surveys largely followed the methodology used at a site of similar size in England by Zhao et al. (2006), and used a gridded sampling pattern. This pattern was initially laid out on a map of each loch. Grid resolution was based on the optimum balance between maximum survey coverage and length of time available; 30 m × 30 m at Upper Glenastle Loch (n = 147), 45 m × 45 m at Tangy Loch (n = 95) and Loch of Butterstone (n = 100). Grid reference with relation to prevailing wind, water depth and percentage cover of each plant species present were recorded at each point. Plant percentage cover was estimated using a bathyscope assisted by two throws of a double headed rake. A surface sediment sample was taken at each point in all lochs following the methodology used by Birks (1973), using an Ekman Grab, which quickly provided a bulk sample of the top 7–10 cm of sediment. This allowed a large volume of sediment to be collected rapidly, and was considered important because previous studies have suggested that sediment volumes of approximately 150cm3 are optimal for macrofossil analysis (Patmore et al. 2014). Furthermore, N. flexilis seeds have been shown to germinate and grow when buried with up to 10 cm of sediment, and samples collected in the Ekman Grab could therefore still represent present plant growth (Wingfield 2002). Birks (1973) describes carefully spooning the top few centimetres of mud from the Ekman sample, but this was not possible at any of the sites in this study due to the unconsolidated nature of the sediments collected. Therefore, to ensure that this approach was not skewed by past N. flexilis seed distributions, three Glew cores of 69.5 mm diameter (Glew 1991) (“BUTT9”, “BUTT10” and “BUTT11”) were additionally taken at Loch of Butterstone (Fig. 2), the top 15 cm of which were extruded at 1cm intervals for analysis. These cores were taken in areas where high numbers of seeds were found in the Ekman grabs, and in areas where N. flexilis plants were found historically. Glew cores were not taken at Upper Glenastle Loch or Loch Tangy because, unlike at Loch of Butterstone (where N. flexilis had recently become extinct), distinction between the numbers of seeds at the top and bottom of the Glew cores was not considered likely.
A measured volume of approximately 100 ml wet sediment from each sample, including the extracted Glew cores was passed through a 355 µm sieve. The retent was transferred into a beaker with some tap water, and in some cases disaggregated using 3 ml of Na6O18P6. Samples were then examined under a Leica dissecting microscope with a 10× magnification setting, and the number of N. flexilis seeds counted.
Distributions of N. flexilis plants and seeds were analysed within sites and across all three sites, and, at Loch of Butterstone, were compared to the historical plant distribution studies of Stewart (1982, pers. commun.), James and Barclay (1996, unpublished) and Murphy (2007). Relationships between seed numbers and N. flexilis percentage cover, total percentage cover, water depth, latitude and longitude were plotted on scatter charts to assess normality and monotony. Subsequently, Kendall’s tau (Kendall 1938) was used to assess correlation between these variables. Mann-Whitney U tests were used to compare N. flexilis plant and seed numbers between lakes. Kernel density analysis (a non-parametric estimation of the probability density function) was used to estimate the probability that a sediment sample with a set number of seeds was taken from each site. All statistical analysis was carried out using R ‘stats’ package version 2.15.3 (R Core Team 2013).
Results
Najas flexilis plants were found at 44% of sample points at Upper Glenastle Loch, 13% at Loch Tangy and at none of the sample points at Loch of Butterstone, the latter in keeping with the absence of N. flexilis in surveys since 2004. Seeds were found in the surface sediments of 97% of sample points at Upper Glenastle Loch, 83% at Tangy Loch and 50% at Loch of Butterstone. Plant and seed distributions at each site are shown in Fig. 3. At Upper Glenastle Loch, plants were primarily distributed in shallower areas of the lake, but there was no similar pattern in the distribution of seeds within the surface sediment. At Tangy Loch, N. flexilis plants were found primarily in the north and west margins of the site, while seeds were found in surface sediments from across the entire lake area. As for Upper Glenastle Loch, the distribution of seeds bore little resemblance to the distribution of the plants, with seed numbers highest towards the eastern end of the lake. At Loch of Butterstone, where N. flexilis were not found in the macrophyte survey, seeds were found across the entire area of the lake with a higher number of seeds found in the western half of the site. In a Kendall’s tau rank correlation test, no correlation was found between N. flexilis percentage cover and the number of N. flexilis seeds in the surface sediments within individual lakes (Upper Glenastle Loch τ = −0.0787, P = 0.2128; τ = −0.0405, P = 0.6373.
The three Glew cores taken from Loch of Butterstone all show a general increase in the number of N. flexilis seeds with increasing sediment depth (Fig 4). No seeds were found in the uppermost 2 cm of sediment of any of the cores. Core BUTT10 contained N. flexilis seeds at a sediment depth of 3 cm.
Figure 5 shows scatterplots comparing the number of N. flexilis seeds to N. flexilis plant percentage cover, total macrophyte cover excluding N. flexilis, water depth and latitude and longitude at Upper Glenastle Loch, Tangy Loch and Loch of Butterstone. For most variables, no correlation was found with the number of N. flexilis seeds. At Upper Glenastle Loch it appears that large numbers of seeds were found at depths of 1–2 m, however this is likely because the majority of sample points at this site were at these depths. There was, however, a significant correlation between seed counts and both latitude and longitude at Tangy Loch (τ = 0.4726, P = 1.64 × 10−10 and τ = −0.2590, P = 0.0005 respectively); this suggests that seeds are clustered together spatially, in this case towards the south-east of the loch. Seed numbers were also significantly correlated with latitude at Loch of Butterstone (τ = −0.2311, P = 0.0028). There were further significant correlations between seed counts and total plant percentage cover (τ = −0.2017, P = 0.0071), and between seed counts and water depth (τ = 0.2017, P = 0.0068) at Tangy Loch.
A Mann–Whitney test revealed that the means of the percentage cover of N. flexilis found at Upper Glenastle Loch and Loch Tangy were significantly different from each other (z = 0.0007; P = 0.9832). Table 1 shows that mean seed counts were highest at Upper Glenastle Loch and lowest at Loch of Butterstone.
Mann–Whitney tests revealed that there was a significant difference in the means of seed counts between all three sites. However, the range of seed counts was highest at Loch of Butterstone and lowest at Upper Glenastle Loch, with the largest numbers of seeds in any sample found at Loch of Butterstone where N. flexilis plants were absent. Kernel density estimates were plotted for the seed counts at each of the three sites (Fig. 6) The intersects shown suggest that, when individual sediment grabs from all sites are considered together, seed counts below 1.37 seeds were most likely to belong to Loch of Butterstone, seed counts between 1.37 and 5.65 most likely to belong to Tangy Loch, and seed counts above 5.65 most likely to belong to Upper Glenastle Loch.
Kernel density plot showing probability distributions of seed counts at each study sites. Intersections between density plot lines are marked with dashed lines. Individual samples with seed counts less than 1.37 (intersect 1) are most likely to be associated with Loch of Butterstone, whilst those with seed counts higher than 5.65 (intersect 2) are most likely to be associated with Upper Glenastle Loch
Discussion
How do N. flexilis seed distributions relate to distributions of parent plants?
Data from all three study lakes suggest that, irrespective of contemporary N. flexilis abundance, seeds tend to be found in large numbers and are distributed across a lake basin. Seeds were also unexpectedly found in 50% of surface sediment samples at Loch of Butterstone, despite the plant being absent from the site. This accords with the findings of Birks (1973), who found N. flexilis macrofossils to be widespread in lakes across the environmental gradient, despite the complete absence of the plant from some sites. Furthermore, within individual lakes, there was no correlation between the numbers of seeds found in surface sediments and the percentage cover of N. flexilis. This implies that N. flexilis seeds are not concentrated close to parent plants. Seed distributions can be partially explained by plant reproductive attributes (Kolar and Lodge 2001; Levin et al. 2003). Many aquatic plants produce seeds that float for a limited time on water, allowing them to be carried by hydrochory (Nilsson et al. 2010). N. flexilis seeds are large, heavy, lack buoyancy and do not have an obvious adaption to dispersal. The wide distributions of N. flexilis seeds are therefore unexpected. It has been suggested that N. flexilis plants, when in seed, may either uproot or fragment and float (Preston and Croft 2001). In this study large numbers of N. flexilis plants were observed on the strandline of Loch Tangy, many with seeds still attached. This “rafting” dispersal mechanism has never been formally measured, but was hypothesised as an explanation for large numbers of Potamogeton seeds being found in surface sediments at Green Plantation Pond, England (Zhao et al. 2006). It may also explain the widespread presence of N. flexilis seeds in areas of lakes where no N. flexilis plants were found, including at Loch of Butterstone, which is hydrologically connected to two sites at which N. flexilis has been observed recently. If this is the case, N. flexilis dispersal patterns will be affected by water currents. Although there is little evidence for this being a driving force at Upper Glenastle Loch, longitude and latitude were found to be strongly correlated with seed numbers at Loch Tangy, with the largest numbers of seeds found towards the eastern end of the loch. This end of the loch is shallower than the western end, explaining the significant correlation between seed numbers and water depth at this site. Tangy Loch is situated in a narrow valley which acts as a funnel for the wind and creates a strong prevailing westerly and thus water currents would carry N. flexilis rafts eastwards. At Loch of Butterstone, seeds were clustered towards the centre and east of the loch; this is likely for similar reasons.
An alternative explanation for the large numbers and variable distributions of seeds found in the surface sediments at Loch of Butterstone is methodological; although the Ekman grab has the advantage of being able to gather a large amount of surface sediment very quickly, it collects amalgamated sediments from the top 7–10 cm and may therefore represent sediments laid down historically. The resolution was originally considered adequate based upon findings that N. flexilis seeds could germinate and grow when buried with up to 10 cm of sediment (Wingfield 2002), and could therefore still present the opportunity for plant growth. Glew cores take a smaller volume of sediment, but can be extruded at much finer intervals—in this case, 1 cm. The numbers of seeds in all three Glew cores from Loch of Butterstone increased with depth, and none of them contained any N. flexilis seeds in the upper 3 cm. Based upon the sediment accumulation rates in an open water core (Bennion et al. 2010), the top 3 cm could represent the period from approximately 2003 to present, and the entire 15cm length of the Glew cores could represent approximately the past 100 years. N. flexilis was last observed at Loch of Butterstone in 2004 (Wingfield et al. 2006), and this aligns with the disappearance of N. flexilis from the Glew cores. Applying the same sedimentation rates, the Ekman grab could collect an amalgamation of sediments laid down since ~1965 and therefore represents the historical distribution of seeds in the sediments. However, it remains clear that, at all three sites, N. flexilis seeds are widely distributed and that seeds are likely to be found regardless of coring location. In fact, the widespread distribution of the seeds could imply that N. flexilis, as an annual, grows in different locations from year to year, and that the location of current stands of the plant can not necessarily be used as a guide when taking cores to investigate past populations. Given that seeds were found in all three Glew cores, it is recommended that, were this study to be repeated, Glew cores be considered instead of Ekman grabs.
In a similar study at Green Plantation Pond, a small shallow English Lake, Zhao et al. (2006) concluded that, in general, macrofossil assemblages in sediments best represent local patch-scale vegetation within 20–30 m of the core site. This pattern was shown to be consistent over a period of 9 years in a follow-up study by Clarke et al. (2014). Potamogeton spp, in particular, seem to be susceptible to this (Davidson et al. 2005; Zhao et al. 2006; Salgado et al. 2010). However, a number of exceptions have been noted for which reproductive sub-fossils were much more widely distributed than contemporary plants. Those species with wide macrofossil distributions include Zannichelia palustris at Green Plantation Pond (Zhao et al. 2006; Clarke et al. 2014) and Najas marina and Characeae in Mediterranean lakes (Levi et al. 2014). All three of these species are known to produce relatively large numbers of oospores/seeds per plant and have an annual (N. marina) or pseudo-annual (Z. palustris and Chara spp.) reproductive strategy (Van Vierssen 1982; Bonis and Grillas 2002; Stace 2010). N. flexilis appears to have similar life strategies.
How does N. flexilis seed abundance relate to percentage cover of parent plants?
Although N. flexilis seed distributions within sites were unrelated to N. flexilis plant distributions, lakes with higher abundances of N. flexilis did harbour larger numbers of N. flexilis seeds than those with lower plant abundances. This may be explained by the concept of “propagule pressure”. Propagule pressure refers to the number of individuals released in any one seed event and the number of discrete release events (Lockwood 2006). Following this, plants that already have a broad distribution and/or produce large numbers of seeds are likely to disperse seeds more efficiently. At Upper Glenastle Loch, where N. flexilis plants are abundant, propagule pressure is higher than at Loch of Butterstone, where there are no N. flexilis plants.
Implications for paleolimnology
It is evident that N. flexilis is adapted for widespread seed dispersal. Thus, macrofossil reconstructions from sediment cores taken from any location within a basin will likely show evidence of present and past populations of N. flexilis. Furthermore, on average, N. flexilis seeds were found in higher numbers in surface sediments at sites where N. flexilis plants were more abundant. This suggests that, where changing trends in N. flexilis seed numbers in a core exist, conclusions can be drawn about the changing abundance of the plant through time. At Upton Great Broad, Norfolk, England, Ayres et al. (2008) successfully used historical records to confirm an increase in another member of the Najadiceae (Najas marina) that was suggested by a large increase in seed numbers towards the top of a sediment core, and the evidence presented in this study suggests that the same is possible for N. flexilis.
Kernel density estimations show that seed counts below 1.37 seeds per 100 mg wet sediment are most likely to represent very small N. flexilis populations, whilst those above 5.65 seeds per 100 mg are most likely to represent widespread, healthy N. flexilis populations. However, there was a very high variation in the numbers of seeds found within each site. Care must therefore be taken not to infer changes in N. flexilis abundance from short term fluctuations in the number of seeds found in the core. As an annual plant with an ability to disperse away from the parent plant, N. flexilis has the potential to change its distribution within a site on an inter-annual basis. Changing numbers of seeds in a single core where no obvious trend is present may simply represent inter-annual changes in the relative importance of factors that influence seed distribution. Further representation studies should be undertaken to expand the number of sites included to reduce the uncertainties associated with the numerical predictions presented here.
Najas flexilis seeds were not only found in the surface sediments of the two sites that supported N. flexilis, but also at Loch of Butterstone where N. flexilis has not been found since 2004. The absence of N. flexilis from the tops of the cores, however, suggests that the Ekman grab samples may over-estimate recent abundance of N. flexilis. Ekman grabs were used in this study to ensure that enough sediment was collected to allow sufficient numbers of N. flexilis seeds in each sample. A common problem with conventional, small bore sediment cores is that, once extruded, they do not contain enough material for macrofossil analysis to be significant (Patmore et al. 2014). This was a problem with the Glew cores in this study where, in most cases, a maximum of 30–40 g wet sediment could be sieved for each sample. However, this study indicates that the Ekman grab is insufficient for further temporal work on the species and lends support to the use of wide diameter cores, such as the Big Ben sediment corer, for paleolimnological studies of N. flexilis and other plants (Patmore et al. 2014).
In accordance with our findings at Loch of Butterstone, a paleoecological investigation of Loch Flemington, Scotland, found N. flexilis seeds in surface sediments despite no present or past records of the plant at the site (Bennion et al. 2008). N. flexilis is notorious for being under-recorded using grapnel techniques (Capers 2000; Wingfield et al. 2005), and it is possible that N. flexilis is present in low numbers both at Loch Flemington and Loch of Butterstone. Although it is highly likely that N. flexilis will be recorded in a sediment core if the plant is present, the combined findings from Loch of Butterstone in this study and Loch Flemington in Bennion et al. (2008) mean that it cannot be assumed that the absence of N. flexilis seeds within a core sample represents the certain absence of N. flexilis plants without further investigation. Paleolimnologists should be cautious of the potential for a “false positive” when N. flexilis seeds are found within a sediment core at sites at which N. flexilis has not been recorded historically.
Several studies have shown that plant remains from different species have different distributions within lake basins (Davidson et al. 2005; Zhao et al. 2006; Salgado et al. 2010; Madgwick et al. 2011). Clarke et al. (2014) recommend that cores therefore be taken close enough to the shore of a lake to pick up as many remains as possible, but not so close that the sediment profile is disturbed. Others have approached the problem of patchy macrophyte distributions by taking multiple cores from lakes. For example, multiple cores taken from Barton Broad, England, demonstrated that Chara oospores were historically present more frequently in the north of the lake than the south (Madgwick et al. 2011). Similarly, Sayer et al. (2010) could determine changes in spatial macrophyte distribution at Felbrigg Hall Lake, Norfolk, England, by comparing macrofossil records in five different sediment cores taken from across the site. In reconstructions of N. flexilis as a single species, such approaches are unnecessary since seeds are highly likely to be found in cores taken from any location within a basin.
Conclusions
Najas flexilis is adapted for dispersal of seeds away from the parent plant, and seeds are distributed widely across lake basins, and is therefore ideal for paleolimnological work since seeds are highly likely to be present in any wide-bore sediment core taken from a basin containing the plant. N. flexilis seed counts are higher on average at sites with a higher abundance of N. flexilis plants, and seed counts above 5.65 seeds per 100mg wet sediment are most likely to represent a widespread, healthy population of N. flexilis. However, the largest number of seeds in a single surface sediment sample in this study was found at Loch of Butterstone, where N. flexilis plants were not present. N. flexilis seeds therefore have the potential to be over-represented in sediment cores, particularly in cases where plant numbers are low. Special care should be taken not to draw spurious conclusions on any changes in N. flexilis abundance that are not based upon clear, consistent trends in seed numbers within sediment cores, ideally backed up by historical records.
Using macrofossils as a proxy for N. flexilis abundance in conjunction with information on dispersal potential may reveal information on the apparent decline of N. flexilis in Scotland over the past 100 years, and in this respect paleolimnological reconstructions are already underway. In addition to presenting important conclusions about the distribution and reproductive ecology of a rare species, this study highlights the value of research at the interface between contemporary ecology and paleoecology. By developing our understanding of the ways in which individual species are represented in sediment cores, conservation science can benefit from the long-term records of environmental change that paleolimnology provides—not just at the community vegetation level, but also regarding individual target species.
Change history
08 September 2017
An erratum to this article has been published.
References
Ayres KR, Sayer CD, Skeate ER, Perrow MP (2008) Paleolimnology as a tool to inform shallow lake management: an example from Upton Great Broad, Norfolk, UK. Biodivers Conserv 17:2153–2168
Bennion H, Clarke G, Davidson D, Morley N, Rose S, Yang H (2008) Palaeoecological study of seven mesotrophic lochs. Environmental Change Research Centre Research Report No. 121
Bennion H, Rawcliffe R, Goldsmith B, Davidson T, Sayer C, Yang H, Rose N (2010) ‘Paleoecological study of two lochs: Butterstone Loch and Lindores Loch.’ Scottish Natural Heritage Commissioned Report No. 355
Biodiversity Action Reporting System (2008) (WWW) Status—Najas flexilis (Slender Naiad). http://ukbars.defra.gov.uk/plans/status.asp?HAP=&SAP={A3C67B92-0A76-41D1-8DED-F269E39E1430 21/02/12
Birks HH (1973) Modern macrofossil assemblages in lake sediments in Minnesota. In: Birks HJB, West RG (eds) Quarternary plant ecology: the 14th symposium of The British Ecological Society, University of Cambridge, 28–30 March 1972. Blackwell Scientific Publishers, Oxford, pp 173–189
Birks HH (2001) Plant macrofossils. In: Smol JP, Birks JB, Last WM (eds) Tracking environmental change using lake sediments vol 3: terrestrial, algal and siliceous indicators. Kluwer: Dordrecht, pp 49–74
Birks HJB (1995) Quantitative palaeoenvironmental reconstructions. In: Maddy D, Brew JS (eds) Statistical modelling of quaternary science data’. Quaternary Research Association, Cambridge, pp 161–254
Blomqvist S (1990) Sampling performance of Ekman grabs: in situ observations and design improvements. Hydrobiologia 206:245–254
Bonis A, Grillas P (2002) Deposition, germination and spatio-temporal patterns of charophyte propagule banks: a review. Aquat Bot 72:235–248
Capers RS (2000) A comparison of two sampling techniques in the study of submersed macrophyte richness and abundance. Aquat Bot 68:87–92
Clarke GH, Sayer CD, Turner S, Salgado J, Meis S, Patmore IR, Zhao Y (2014) Representation of aquatic vegetation change by plant macrofossils in a small and shallow freshwater lake. Veg Hist Archaeobot 23:265–276
Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and of Wild Fauna and Flora
Davidson TA, Sayer CD, Bennion H, David C, Rose N, Wade MP (2005) A 250 year comparison of historical, macrofossil and pollen records of aquatic plants in a shallow lake. Freshw Biol 50:1671–1686
Dudgeon D, Arthington AH, Gessner MO, Kawabata Z, Knowler DJ, Lévêque C, Naiman RJ, Prieur-Richard A, Soto D, Staissny MLJ, Sullivan CA (2006) Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev (Camb) 81:163–182
Ekman S (1911) Neue apparate zur qualitativen Erforschung der Bodenfauna der Seen. Hydrobiologia 3:553–561
Finney N (1998) A nutrient history of the Lunan Burn lochs, central Scotland. Thesis submitted for MRes Environmental Sciences, UCL
Gałka M, Tobolski K, Kołaczek P (2012) The Holocene decline of Slender Naiad (Najas flexilis (Willd.) Rostk. & W. L. E Schmidt) in NE Poland in the light of new palaeobotanical data. Acta Palaeobot 52:127–138
Glew JR (1991) Miniature gravity corer for recovering short sediment cores. J Paleolimnol 5:285–287
Hutchinson GE (1975) A treatise on limnology. Wiley, New York
Kendall M (1938) A new measure of rank correlation. Biometrika 30(1–2):81–89
Kolar CS, Lodge DM (2001) Progress in invasion ecology: predicting invaders. Trends Ecol Evol 16(4):199–204
Levi EE, Cakiroĝlu AI, Bucak T, Odgaard BV, Davidson TA, Jeppesen E, Beklioĝlu M (2014) Similarity between contemporary vegetation and plant remains in the surface sediment in Mediterranean lakes. Freshw Biol 59:724–736
Lockwood JL (2006) The role of propagule pressure in explaining species invasions. Trends Ecol Evol 20(5):223–228
Loreau M, Naeem S, Inchausti P, Bergtsson P, Grime JP, Hector A, Hooper DU, Huston MA, Raffaelli P, Schmid B, Tilman D, Wardle DA (2001) Biodiversity and ecosystem functioning: current knowledge and future challenges. Science 294:804–808
MacArthur RH, Wilson EO (1967) The theory of island biogeography. Princeton University Press, Princeton
Madgwick G, Emson D, Sayer CD, Willby NJ, Rose NL, Jackson MJ, Kelly A (2011) Centennial-scale changes to the aquatic vegetation structure of a shallow eutrophic lake and implications for restoration. Freshw Biol 56:2620–2636
Moyle JB (1945) Some chemical factors influencing the distribution of aquatic plants in Minnesota. Am Midl Nat 34:402–420
National Biodiversity Network (2014) (WWW) NBN Gateway. http://data.nbn.org.uk/ 20/4/2014
O Connor Á (2013) Article 17 assessment form and audit trail for Najas flexilis, the Slender Naiad (species code 1833). Backing document, April 2013. National Parks and Wildlife Service, Department of Arts, Heritage and the Gaeltacht, Ireland
Patmore IR, Sayer CD, Goldsmith B, Davidson TA, Rawcliffe R, Salgado J (2014) Big ben: a new wide-bore piston corer for multi-proxy paleolimnology. J Paleolimnol 51:79–86
Pip E, Simmons K (1986) Aquatic angiosperms at unusual depths in Shoal Lake, Manitoba, Ontario. Can Field-Nat 100:354–358
Preston CD, Croft JM (1997) Aquatic plants of Britain and Ireland. Harley Books, Colchester
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
RenSmart (2016) (WWW) Wind Data Archive. http://www.rensmart.com/Weather/WindArchive 30/8/16
Rondinini C, Wilson KA, Boitani L, Grentham H, Possingham HP (2006) Tradeoffs of different types of species occurrence data for use in systematic conservation planning. Ecol Lett 9:1136–1145
Salgado J, Sayer CD, Carvalho L, Davidson TA, Gunn I (2010) Assessing aquatic macrophyte community change through the integration of paleolimnology and historical data at Loch Leven, Scotland. J Paleolimnol 43:191–204
Sayer CD, Burgess A, Kari K, Davidson TA, Peglar S, Yang H, Rose N (2010) Long-term dynamics of submerged macrophytes and algae in a small and shallow eutrophic lake: Implications for the stability of macrophyte dominance. Freshw Biol 55:565–583
Stace CA (2010) New flora of the British Isles, 3rd edn. Cambridge University Press, Cambridge
Sutherland WJ, Pullin AS, Dolman PM, Knight TM (2004) The need for evidence-based conservation. Trends Ecol Evol 19(6):305–308
UNEP (2000) Sustaining Life on Earth: How The Convention on Biological Diversity Promotes Nature and Human Well-being. Secretariat of the Convention of Biological Diversity
Van Vierssen W (1982) The ecology of communities dominated by Zannichellia taxa in Western Europe. I. Characterisation and autoecology of the Zannichellia taxa. Aquat Bot 12:103–155
Wingfield RG (2002) The functional ecology of Najas flexilis. Ph.D. Thesis, University of Glasgow
Wingfield R, Murphy KJ, Gaywood M (2005) Lake habitat suitability for the rare European macrophyte Najas flexilis (Willd.) Rostk. & Schmidt. Aquat Conserv 15:227–241
Wingfield R, Murphy KJ, Gaywood M (2006) Assessing and predicting the success of Najas flexilis (Willd.) Rostk & Schmidt, a rare European aquatic macrophyte, in relation to lake environmental conditions. Hydrobiologia 570(1):79–86
Wingfield, RA, Murphy, KJ, Hollingsworth, P, Gaywood, MJ (2004) The ecology of Najas flexilis. Scottish Natural Heritage Commissioned Report No. 017
Zhao Y, Sayer CD, Birks HH, Hughes M, Peglar S (2006) Spatial representation of aquatic vegetation by macrofossils and pollen in a small shallow lake. J Paleolimnol 35:335–350
Acknowledgements
This research was supported by grants from the Botanical Society of the British Isles (BSBI), Scottish Natural Heritage (SNH), Scottish Environmental Protection Agency (SEPA) and Memset. Help with field and laboratory work was also gratefully received from Janet Hope, Kate Craig-Wood, Wayne Smith, Paul Taylor and Chris Palmer.
Author information
Authors and Affiliations
Corresponding author
Additional information
The original version of this article was revised: Figure 5 is revised.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bishop, I.J., Bennion, H., Patmore, I.R. et al. How effective are plant macrofossils as a proxy for macrophyte presence? The case of Najas flexilis in Scotland. J Paleolimnol 60, 153–165 (2018). https://doi.org/10.1007/s10933-017-9988-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10933-017-9988-5