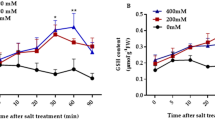
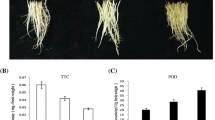
Abstract
The thiol–disulphide exchange regulates the activity of proteins by redox modulation. Many studies to analyze reactive oxygen species (ROS), particularly, hydrogen peroxide (H2O2) induced changes in the gene expression have been reported, but efforts to detect H2O2 modified proteins are comparatively few. Two-dimensional diagonal redox sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS PAGE) was used to detect polypeptides which undergo thiol–disulphide exchange in Brassica juncea seedlings following H2O2 (10 mM) treatment for 30 min. Eleven redox responsive polypeptides were identified which included cruciferin, NLI [Nuclear LIM (Lin11, Isl-1 & Mec-3 domains)] interacting protein phosphatase, RuBisCO (ribulose-1,5-bisphosphate carboxylase/oxygenase) large subunit, and myrosinase. Redox modulation of RuBisCO large subunit was further confirmed by western blotting. However, the small subunit of RuBisCO was not affected by these redox changes. All redox modulated targets except NLI interacting protein (although it contains two cysteines) showed oxidation sensitive cysteines by in silico analysis. Interestingly, interactome of cruciferin and myrosinase indicated that they may have additional function(s) beside their well-known roles in the seedling development and abiotic stress respectively. Cruciferin showed interactions with stress associated proteins like defensing-like protein 192 and 2-cys peroxiredoxin. Similarly, myrosinase showed interactions with nitrilase and cytochrome p450 which are involved in nitrogen metabolism and/or hormone biosynthesis. This simple procedure can be used to detect major stress mediated redox changes in other plants.




Similar content being viewed by others
Abbreviations
- ROS:
-
Reactive oxygen species
- H2O2 :
-
Hydrogen peroxide
- 2D Diagonal Redox SDS PAGE:
-
2-Dimensional redox sodium dodecyl sulfate polyacrylamide gel electrophoresis
- RuBisCO:
-
Ribulose-1, 5-bisphosphate carboxylase/oxygenase
References
Xie LH, Chen F, Karagueuzian HS, Weiss JN (2009) Oxidative stress-induced after depolarizations and calmodulin kinase II signaling. Circ Res 104:79–86
Hasanuzzaman M, Nahar K, Alam MM, Roychowdhury R, Fujita M (2013) Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci 14(5):9643–9684
Hossain MA, Bhattacharjee S, Armin SM, Qian P, Wang X, Li HY, Burritt DJ, Fujita M, Tran LS (2015) Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Front Plant Sci 6:420
Janssen-Heininger YMW, Aesif SW, Velden J, Guala AS, Reiss JN, Roberson EC, Budd RC, Reynaert NL, Anathy V (2010) Regulation of apoptosis through cysteine oxidation: implications for fibrotic lung disease. Ann N Y Acad Sci 1203:23–28
García-Santamarina S, Boronat S, Hidalgo B (2014) Reversible cysteine oxidation in hydrogen peroxide sensing and signal transduction. BioChemistry 53:2560–2580
Rinalducci S, Murgiano L, Zolla L (2008) Redox proteomics: basic principles and future perspectives for the detection of protein oxidation in plants. J Exp Bot 59:781–801
Nadeau PJ, Charette SJ, Toledano MB, Landry J (2007) Disulfide bond-mediated multimerization of Ask1 and its reduction by thioredoxin-1 regulate H2O2-induced c-Jun NH2-terminal kinase activation and apoptosis. Mol Biol Cell 18:3903–3913
Reczek CR, Chandel NS (2015) ROS-dependent signal transduction. Cur Opin Cell Biol 33:8–13
Stroher E, Dietz KJ (2008) The dynamic thiol–disulphide redox proteome of the Arabidopsis thaliana chloroplast as revealed by differential electrophoretic mobility. Physiol Plant 133:566–583
Sethuraman M, Comb Mc, Huang ME, Huang H, Heibeck T, Costello CE, Cohen RA (2004) Isotope-coded affinity tag (ICAT) approach to redox proteomics: identification and quantitation of oxidant-sensitive cysteine thiols in complex protein mixtures. J Proteome Res 6:1228–1233
Liu P, Zhang H, Wang H, Xia Y (2014) Identification of redox-sensitive cysteines in the Arabidopsis proteome using OxiTRAQ, a quantitative redox proteomics method. Proteomics 14:750–762
Wong JH, Balmer Y, Cai N, Tanaka CK, Vensel WH, Hurkman WJ, Buchanan BB (2003) Unraveling thioredoxin-linked metabolic processes of cereal starchy endosperm using proteomics. FEBS Lett 547:151–156
Wong JH, Cai N, Balmer Y, Tanaka CK, Vensel WH, Hurkman WJ, Buchanan BB (2004) Thioredoxin targets of developing wheat seeds identified by complementary proteomic approaches. Phytochemistry 65:1629–1640
Alkhalfioui F, Renard M, Vensel WH, Wong J, Tanaka CK, Hurkman WJ, Buchanan BB, Montrichard F (2007) Thioredoxin-linked proteins are reduced during germination of Medicago truncatula seeds. Plant Physiol 144:1559–1579
Bykova NV, Hoehn B, Rampitsch C, Hu J, Stebbing JA, Knox R (2011) Thiol redox-sensitive seed proteome in dormant and non-dormant hybrid genotypes of wheat. Phytochemistry 10:1162–1172
Alvarez S, Zhu M, Chen S (2009) Proteomics of Arabidopsis redox proteins in response to methyl jasmonate. J Proteomics 73:30–40
Wang H, Wang S, Lu Y, Alvarez S, Hicks LM, Ge X, Xia Y (2012) Proteomic analysis of early-responsive redox-sensitive proteins in Arabidopsis. J Proteome Res 11:412–424
Galant A, Koester RP, Ainsworth EA, Hicks LM, Jez JM (2012) From climate change to molecular response: redox proteomics of ozone-induced responses in soybean. New Phytol 1:220–229
Hägglund P, Bunkenborg J, Maeda K, Svensson B (2008) Identification of thioredoxin disulfide targets using a quantitative proteomics approach based on isotope-coded affinity tags. J Proteome Res 12:5270–5276
Sehrawat A, Deswal R (2014) S-nitrosylation analysis in Brassica juncea apoplast highlights the importance of nitric oxide in cold-stress signaling. J Proteome Res 7(12):2599–2619
Muthuramalingam M, Matros A, Scheibe R, Hans-Peter M, Karl-Josef D (2013) The hydrogen peroxide-sensitive proteome of the chloroplast in vitro and in vivo. Front Plant Sci 4:1–14
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A 76:4350–4354
EDBCP tool. http://omictools.com. Accessed 11 Aug 2016
DiANNA 1.1 web server. http://clavius.bc.edu. Accessed 11 Aug 2016
CYS_REC program. http://www.softberry.com. Accessed 16 Aug 2016
Trujillo M, Alvarez B, Radi R (2015) One- and two-electron oxidation of thiols: mechanisms, kinetics and biological fates. Free Radic Res 50:150–171
Poole LB (2015) The basics of thiols and cysteines in redox biology and chemistry. Free Radic Biol Med 0:148–157
Lin HH, Hsu J-C, Hsu Y-N, Pan R-H, Chen Y-F, Tseng L-Y (2013) Disulfide connectivity prediction based on structural information without a prior knowledge of the bonding state of cysteines. Comput Biol Med 43:941–1948
Jones DT (1999) Protein secondary structure prediction based on position-specific scoring matrices. J Mol Biol 292:195–202
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Ferrè F, Clote P (2005) Disulfide connectivity prediction using secondary structure information and diresidue frequencies. Bioinformatics 21:2336–2346
Principle of DiANNA (2016) Softberry Inc, USA. http://linux1.softberry.com. Accessed 16 Aug 2016
Wittstocka U, Burowb M (2010) Glucosinolate breakdown in Arabidopsis: mechanism, regulation and biological significance. Arabidopsis Book. doi:10.1199/tab.0134
Yano H, Wong JH, Cho MJ, Bob B (2001) Redox changes accompanying the degradation of seed storage proteins in germinating rice. Plant Cell Physiol 42:879–883
Bassham J, Benson A, Calvin M (1950) The path of carbon in photosynthesis. J Biol Chem 185:781–787
Berg JM, Tymoczko JL, Stryer L (2002) Biochemistry, 5th edn. W H Freeman, New York
García-Ferris C, Moreno J (1994) Oxidative modification and breakdown of ribulose 1,5-bisphosphate carboxylase/oxygenase induced in Euglena gracilis by nitrogen starvation. Planta 193:208–215
Ferreira RB, Davies DD (1989) Conversion of ribulose-1,5-bisphosphate carboxylase to an acidic and catalytically inactive form by extracts of osmotically stressed Lemna fronds. Planta 179:448–455
García-Ferris C, Moreno J (1993) Redox regulation of enzymatic activity and proteolytic and susceptibility of ribulose-1,5-bisphosphate carboxylase/oxygenase from Euglena gracilis. Photosynth Res 35:55–66
Moreno J, Penarrubia L, Garcia-Ferris C (1995) The mechanism of redox regulation of ribulose-1,5-bisphosphate carboxylase/oxygenase turnover. A hypothesis. Plant Physiol Biochem 33:121–127
Schloss JV, Stringer CD, Hartman FC (1978) Identification of essential lysyl and cysteinyl residues in spinach ribulosebisphosphate carboxylase/oxygenase modified by the affinity label N-bromoacetylethanolamine phosphate. J Biol Chem 253:5707–5711
Julia M-N, Moreno J (2006) Cysteines 449 and 459 modulate the reduction–oxidation conformational changes of ribulose 1·5-bisphosphate carboxylase/oxygenase and the translocation of the enzyme to membranes during stress. Plant Cell Environ 29:898–908
Moreno J, García-Murria MJ, Marín-Navarro J (2008) Redox modulation of Rubisco conformation and activity through its cysteine residues. J Exp Bot 59:1605–1614
Marcus Y, Altman-Gueta H, Finkler A, Gurevitz M (2003) Dual role of cysteine 172 in redox regulation of ribulose 1,5-bisphosphate carboxylase/oxygenase activity and degradation. J Bacteriol 85:1509–1517
Abat JK, Mattoo AK, Deswal R (2008) S-nitrosylated proteins of a medicinal CAM plant Kalanchoe pinnata—ribulose-1,5-bisphosphate carboxylase/oxygenase activity targeted for inhibition. FEBS J 275:2862–2872
Abat K, Deswal R (2009) Differential modulation of S-nitrosoproteome of Brassica juncea by low temperature: change in S-nitrosylation of Rubisco is responsible for the inactivation of its carboxylase activity. Proteomics 9:4368–4380
Job C, Rajjou L, Lovigny Y, Belghazi M, Job D (2005) Patterns of protein oxidation in Arabidopsis seeds and during germination. Plant Physiol 138:790–802
Thomas N, Dudkina NV, Haase C, Denolf P, Semchonok DA, Boekema EJ, Braun HP, Sunderhaus S (2013) The native structure and composition of the Cruciferin complex in Brassica napus. J Biol Chem 288:2238–2245
Bernardi R, Finiguerra MG, Rossi AA, Palmieri S (2003) Isolation and biochemical characterization of a basic myrosinase from ripe Crambe abyssinica seeds, highly specific for epi-progoitrin. J Agric Food Chem 51:2737–2744
Burgmeister W, Cottaz S, Driguez H, Iori R, Palmieri S, Henrissat B (1997) The crystal structures of Sinapis alba myrosinase and a covalent glycosyl-enzyme intermediate provide insights into the substrate recognition and active-site machinery of an S-glycosidase. Structure 5:663–675
Jurata LW, Pfaff SL, Gill GN (1998) The nuclear LIM domain interactor NLI mediates homo- and heterodimerization of LIM domain transcription factors. J Biol Chem 273:3152–3157
Acknowledgements
Funding for this work was provided by University Grant Commission (UGC), India in the form of Non-Net fellowship and by University of the Delhi, Research & Development Grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical Approval
Antibodies were raised using two male rabbits following guidelines from the Canadian Council on Animal Care.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chaurasia, S.P., Deswal, R. Identification and In Silico Analysis of Major Redox Modulated Proteins from Brassica juncea Seedlings Using 2D Redox SDS PAGE (2-Dimensional Diagonal Redox Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis). Protein J 36, 64–76 (2017). https://doi.org/10.1007/s10930-017-9698-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-017-9698-x