Abstract
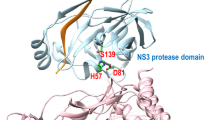
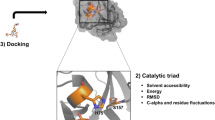
Hepatitis C Virus (HCV) non-structural protein 3 (NS3) protease drug resistance poses serious challenges on the design of an effective treatment. Substrate Envelope Hypothesis, “the substrates of HCV NS3/4A protease have a consensus volume inside the active site called substrate envelope” is used to design potent and specific drugs to overcome this problem. Using molecular docking, we studied the binding interaction of the different inhibitors and protein and evaluated the effect of three different mutations (R155K, D168A and A156V) on the binding of inhibitors. P2–P4 macrocycles of 5A/5B and modified 5A/5B hexapeptide sequences have the best scores against the wild-type protein −204.506 and −206.823 kcal/mole, respectively. Also, charged P2–P4 macrocycles of 3/4A and 4A/4B hexapeptide sequences have low scores with the wild-type protein −200.467 and −203.186 kcal/mole, respectively. R155K mutation greatly affects the conformation of the compounds inside the active site. It inverts its orientations, and this is because the large and free side chain of K155 which restricts the conformation of the large P2–P4 macrocycle. The conformation of charged P2–P4 macrocycle of 3/4A hexapeptide sequence in wild-type, A156V and D168A proteins is nearly equal; while that of charged P2–P4 macrocycle of 4A/4B hexapeptide sequence is different. Nevertheless, these compounds have a slight increase of Van der Waals volume compared to that of substrates, they are potent against mutations and have good scores. Therefore, the suggested drugs can be used as an effective treatment solving HCV NS3/4A protease drug resistance problem.








Similar content being viewed by others
Abbreviations
- HCV:
-
Hepatitis C Virus
- NS3:
-
Non-structural protein 3
- NS4A:
-
Non-structural protein 4A
- FDA:
-
Food and drug administration
- PEG IFN-α:
-
Pegylated interferon α
- HIV-1:
-
Human immunodeficiency virus-1
- PM3:
-
Parameterization model, version 3
- MM3:
-
Molecular mechanics 3
- MO-G:
-
Molecular orbital package
- NS:
-
Non-structural protein
- PMF:
-
Potential of mean force
References
Pawlotsky JM (2012) Is hepatitis virus resistance to antiviral drugs a threat? Gastroenterology 142:1369–1372
Vermehren J, Sarrazin C (2012) The role of resistance in HCV treatment. Best Pract Res Clin Gastroenterol 26(4):487–503
Welsch C, Zeuzem S (2012) Clinical relevance of HCV antiviral drug resistance. Curr Opin Virol 2(5):651–655
Kairys V, Gilson MK, Lather V, Schiffer CA, Fernandes MX (2009) Toward the design of mutation-resistant enzyme inhibitors: further evaluation of the substrate envelope hypothesis. Chem Biol Drug Des 74:234–245
Romano KP, Ali A, Royer WE, Schiffer CA (2010) Drug resistance against HCV NS3/4A inhibitors is defined by the balance of substrate recognition versus inhibitor binding. Proc Natl Acad Sci 107(49):20986–20991
Romano KP, Laine JM, Deveau LM, Cao H, Massi F, Schiffer CA (2011) Molecular mechanisms of viral and host cell substrate recognition by hepatitis C virus NS3/4A protease. J Virol 85(13):6106–6116
Pan D, Xue W, Zhang W, Liu H (1820) Yao X (2012) Understanding the drug resistance mechanism of hepatitis C virus NS3/4A to ITMN-191 due to R155 K, A156 V, D168A/E mutations: a computational study. Biochim Biophys Acta 10:1526–1534
Romano KP, Ali A, Aydin C, Soumana D, Ozen A, Deveau LM, Silver C, Cao H, Newton A, Petropoulos CJ, Huang W, Schiffer CA (2012) The molecular basis of drug resistance against hepatitis C virus NS3/4A protease inhibitors. PLoS Pathog 8(7):e1002832. doi:10.1371/journal.ppat.1002832
Xue W, Wang M, Jin X, Liu H, Yao X (2012) Understanding the structural and energetic basis of inhibitor and substrate bound to the full-length NS3/4A: insights from molecular dynamics simulation, binding free energy calculation and network analysis. Mol Bio Syst 8:2753–2765
Manns MP, Foster GR, Rockstroh JK, Zeuzem S, Zoulim F, Houghton M (2007) The way forward in HCV treatment–finding the right path. Nat Rev Drug Discov 6:991–1000
Raney KD, Sharma SD, Moustafa IM, Cameron CE (2010) Hepatitis C virus non-structural protein 3 (HCV NS3): a multifunctional antiviral target. J Biol Chem 285(30):22725–22731
Lin C (2006) HCV NS3-4A Serine Protease a book chapter in Hepatitis C viruses: genomes and molecular biology. In: Tan S (ed), Horizon Bioscience, p 163-206
Chen KX, Njoroge FG (2010) The journey to the discovery of Boceprevir: an NS3-NS4 HCV protease inhibitor for the treatment of chronic hepatitis C. Prog Med Chem 49:1–36
Chevaliez S, Pawlotsky JM (2007) Interferon-based therapy of hepatitis C. Adv Drug Deliv Rev 59:1222–1241
Avolio S, Summa V (2010) Advances in the development of macrocyclic inhibitors of hepatitis C virus NS3-4A protease. Curr Top Med Chem 10:1403–1422
Venkatraman S, Njoroge FG (2009) Macrocyclic inhibitors of HCV NS3 protease. Expert Opin Ther Patents 19(9):1277–1303
Elfiky AA, Elshemey WM, Gawad WA, Desoky OS (2013) Molecular modeling comparison of the performance of NS5b polymerase inhibitor (PSI-7977) on prevalent HCV genotypes. Protein J 32(1):75–80
Saleh NA, Elfiky AA, Ezat AA, Elshemey WM, Ibrahim M (2014) The electronic and QSAR properties of modified Telaprevir compounds as HCV NS3 protease inhibitors. J Comp Theo NanoSci 11:1–5
Ibrahim M, Saleh NA, Elshemey WM, Elsayed AA (2012) Hexapeptide functionality of cellulose as NS3 protease inhibitors. Med Chem 8:6–10
Ibrahim M, Saleh NA, Elshemey WM, Elsayed AA (2012) Fullerene derivative as anti-HIV protease inhibitor: molecular modeling and QSAR approaches. Mini Rev Med Chem 12(6):447–451
Huang SY, Zou X (2010) Advances and challenges in protein-ligand docking. Int J Mol Sci 11:3016–3034
Stewart JJP (2009) SCIGRESS, Version 2.9.0, Fujitsu Limited, United States
Ganguly S, Bahare RS (2013) Molecular docking studies of novel thiazolidinedione analogs as HIV-1-RT inhibitors. Med Chem Res 22:3350–3363
Mostafa HIA, El-bialy NS, Ezat AA, Saleh NA, Ibrahim MA, QSAR analysis and molecular docking simulation of suggested Peptidomimetic NS3 Protease Inhibitors. Curr Comp aided Drug Des (in press)
Schiering N, Arcy AD, Villard F, Simić O, Kamke M, Monnet G, Hassiepen U, Svergun DI, Pulfer R, Eder J, Raman P, Bodendorf U (2011) A macrocyclic HCV NS3/4A protease inhibitor interacts with protease and helicase residues in the complex with its full-length target. Proc Natl Acad Sci 108(52):21052–21056
Kroemer RT (2007) Structure-based drug design: docking and scoring. Curr Prot Pep Sci 8:312–328
Muegge I (2006) PMF scoring revisited. J Med Chem 49:5895–5902
http://poseview.zbh.uni-hamburg.de/poseview. Accessed 22 Nov 2013
Steinkühler C, Biasiol G, Brunetti M, Urbani A, Koch U, Cortese R, Pessi A, Francesco RD (1998) Product inhibition of the hepatitis C virus NS3 protease. Biochemistry 37:8899–8905
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ezat, A.A., El-Bialy, N.S., Mostafa, H.I.A. et al. Molecular Docking Investigation of the Binding Interactions of Macrocyclic Inhibitors with HCV NS3 Protease and its Mutants (R155K, D168A and A156V). Protein J 33, 32–47 (2014). https://doi.org/10.1007/s10930-013-9538-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-013-9538-6