Abstract
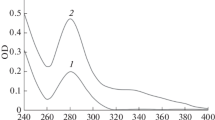
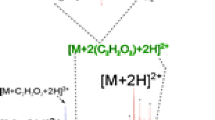
Methylglyoxal (MG), a reactive α-oxoaldehyde, reacts with proteins to form irreversible advanced glycation end products (AGEs) following Maillard-like reaction. MG-induced AGE (MAGE) formation may be significant, particularly in diabetic condition with increased level of MG. Although myoglobin (Mb) is known to react with sugars to form AGEs, its interaction with MG is not known. Here we have studied interaction of Mb with MG. After in vitro reaction between Mb and MG at 25 °C for 7 days, the unchanged Mb and modified Mb (MG-Mb) were separated by ion exchange chromatography. Compared to Mb, MG-Mb exhibited higher electrophoretic mobility in native polyacrylamide gel electrophoresis, increased absorbance around 280 nm and more α-helical content, indicating structural changes of the modified protein. As shown by MALDI-mass spectrometry, MG converted Lys-16 and Lys-133 to carboxyethyllysine in MG-Mb. MAGE thus formed in MG-Mb may be associated with its enhanced mobility in native gel due to neutralization of positive charges and the observed structural changes in comparison with Mb.




Similar content being viewed by others
Abbreviations
- AGEs:
-
Advanced glycation end products
- CD:
-
Circular dichroic
- CEL:
-
Carboxyethyllysine
- CID:
-
Collision-induced dissociation
- MAGE:
-
Methylglyoxal-induced advanced glycation end products
- MALDI-TOF:
-
Matrix-assisted laser-desorption ionization-time of flight
- Mb:
-
Myoglobin
- MG:
-
Methylglyoxal
- MG-H1:
-
Hydroimidazolone
- MG-Mb:
-
Methylglyoxal-modified myoglobin
- MS:
-
Mass spectrometry
- PAGE:
-
Polyacrylamide gel electrophoresis
- PB:
-
Potassium phosphate buffer
References
Ahmed N, Dobler D, Dean M, Thornalley PJ (2005) J Biol Chem 280:5724–5732
Bhattacherjee A, Chakraborti AS (2011) Int J Biol Macromol 48:202–209
Bokiej M, Livermore AT, Harris AW, Onishi AC, Sandwick RK (2011) Biochem Biophys Res Com 407:191–196
Bose T, Bhattacherjee A, Banerjee S, Chakraborti AS (2013) Arch Biochem Biophys 529:99–104
Bose T, Chakraborti AS (2008) Biochim Biophys Acta 1780:800–808
Chaplen FWR, Fahl WE, Cameron DC (1996) Cytotechnology 22:33–42
Chen Y, Ahmed N, Thornalley PJ (2005) Ann NY Acad Sci 1043:905
Chen YH, Yan JT, Martinez HM (1972) Biochemistry 11:4120–4131
Cooper RA (1975) Methods Enzymol 41:535–541
Gallet X, Charloteaux B, Thomas A, Brasseur R (2000) J Mol Biol 302:917–926
Gao Y, Wang Y (2006) Biochemistry 45:15654–15660
Giardino I, Edelstein D, Brownlee M (1994) J Clin Invest 94:110–117
Gomes RA, Miranda HV, Silva MS, Graca G, Coelho AV, Ferreira AE, Cordeiro C, Freire AP (2006) FEBS J 273:5273–5287
Gomes RA, Oliveira LMA, Silva M, Ascenso C, Quintas A, Costa G, Coelho AV, Silva MS, Ferreira AEN, Freire AP, Cordeiro C (2008) Biochem J 416:317–326
Jia X, Olson DJH, Ross ARS, Wu L (2006) Faseb J 20:1555–1557
Kalapos MP (1999) Toxicol Lett 110:145–175
Kang JH (2006) J Biochem Mol Biol 39:335–338
Kumar MS, Reddy PY, Kumar PA, Surolia T, Reddy GB (2004) Biochem J 379:273–282
Lo TWC, Westwood ME, McLellan AC, Selwood T, Thornalley PJ (1994) J Biol Chem 269:32299–32305
Lu J, Randell E, Han Y, Adeli K, Krhan J, Meng QH (2011) Clin Biochem 44:307–311
Nagaraj RH, Shipanova IN, Faust FM (1996) J Biol Chem 271:19338–19345
Nakano S, Mugikura M, Endoh M, Ogami Y, Isuki MJ (1996) J Gastroenterol 31:623–626
Nemet I, Varga-Defterdarovic L, Turk Z (2004) Clin Biochem 37:875–881
Odani H, Shinzato T, Usami J, Matsumoto Y, Frye EB, Baynes JW, Maeda K (1998) FEBS Lett 427:381–385
Oliviera LMA, Lages A, Gomes RA, Neves H, Familia C, Coelho AV, Quintas A (2011) BMC Biochem 12:41
Oya-Ito T, Naitu Y, Takagi T, Handa O, Matsui H, Yamada M, Shima K, Yoshikawa T (2011) Biochim Biophys Acta 1812:769–781
Puttaiah S, Biswas A, Staniszewska M, Nagaraj RH (2007) Exp Eye Res 84:914–921
Ramasamy R, Yan SF, Schmidt AM (2006) Cell 124:258–260
Roy A, Sen S, Chakraborti AS (2004) Free Radic Res 38:139–146
Roy A, Sil R, Chakraborti AS (2010) Mol Cell Biochem 338:105–114
Schalkwijk CG, van Bezu J, van der Schors RC, Uchida K, Stehouwer CD, van Hinsbergh VW (2006) FEBS Lett 580:1565–1570
Sen S, Kar M, Roy A, Chakraborti AS (2005) Biophys Chem 113:289–298
Tanabashi S, Okuno F, Terakura T, Tsuji T, Wakahara T, Yamada S (1982) Nippon Naika Gakki Zasshi 71:802–809
Thornalley PJ (1993) Mol Aspects Med 14:287–371
Wittenberg JB, Wittenberg BA (1981) Methods Enzymol 76:29–42
Acknowledgments
S.B received a research fellowship [No. 09/028(0802)/2010-EMR-1] from the Council of Scientific and Industrial Research, New Delhi. The study was supported by financial assistances from the Department of Science and Technology, New Delhi [Grant No. DST/SR/FST/LSI-286/2006(c)] and the University Grants Commission, New Delhi [Grant No.UGC (DSA) F.4-1/2009 (SAP-II)].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Banerjee, S., Chakraborti, A.S. In Vitro Study on Structural Alteration of Myoglobin by Methylglyoxal. Protein J 32, 216–222 (2013). https://doi.org/10.1007/s10930-013-9480-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-013-9480-7