Abstract
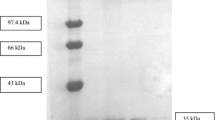
A mutant of the lipase from Geobacillus sp. strain T1 with a phenylalanine to leucine substitution at position 16 was overexpressed in Escherichia coli strain BL21(De3)pLysS. The crude enzyme was purified by two-step affinity chromatography with a final recovery and specific activity of 47.4 and 6,315.8 U/mg, respectively. The molecular weight of the purified F16L lipase was approximately 43 kDa by 12% SDS-PAGE analysis. The F16L lipase was demonstrated to be a thermophilic enzyme due its optimum temperature at 70 °C and showed stability over a temperature range of 40–60 °C. The enzyme exhibited an optimum pH 7 in phosphate buffer and was relatively stable at an alkaline pH 8–9. Metal ions such as Ca2+, Mn2+, Na+, and K+ enhanced the lipase activity, but Mg2+, Zn2+, and Fe2+ inhibited the lipase. All surfactants tested, including Tween 20, 40, 60, 80, Triton X-100, and SDS, significantly inhibited the lipolytic action of the lipase. A high hydrolytic rate was observed on long-chain natural oils and triglycerides, with a notable preference for olive oil (C18:1; natural oil) and triolein (C18:1; triglyceride). The F16L lipase was deduced to be a metalloenzyme because it was strongly inhibited by 5 mM EDTA. Moderate inhibition was observed in the presence of PMSF at a similar concentration, indicating that serine residues are involved in its catalytic action. Further, the activity was not impaired by water-miscible solvents, including methanol, ethanol, and acetone.










Similar content being viewed by others
Abbreviations
- DTT:
-
Dithiothreitol
- EDTA:
-
Ethylenediaminetetraacetic acid
- GST:
-
Glutathione S transferase
- IPTG:
-
Isopropyl β-D-1-thiogalactopyranoside
- PCMB:
-
p-Chloromercuribenzoic acid
- PMSF:
-
Phenylmethanesulfonylfluoride
- PBS:
-
Phosphate buffered saline
- SDS PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
References
Castro-Ochoa LD, Rodrı′guez-Go′mez C, Valerio-Alfaro G, Oliart- Ros RM (2005) Enzyme Microb Technol 37:648–654
Dutta S, Ray L (2009) Appl Biochem Biotechnol 159:142–154
Gutteridge A, Thornton J (2005) J Mol Biol 346:21–28
Jaeger KE, Dijkstra BW, Reetz MT (1999) Annu Rev Microbiol 53:315–351
Jensen RG (1983) Lipids 18:650–657
Kambourova M, Kirilova N, Mandeva R, Derekova A (2003) J Mol Catal B Enzymatic 22:307–313
Kim HK, Park SY, Lee JK, Oh TK (1998) Biosci Biotech Biochem 62(1):66–71
Kim MH, Kim HK, Lee JK, Park SY, Oh TK (2000) Biosci Biotechnol Biochem 64:280–286
Koshland D (1963) Science 142:1533–1541
Kumar S, Kikon K, Upadhyay A, Kanwar SS, Gupta R (2005) Protein Expr Purif 41(1):38–44
Kwon DY, Rhee JS (1986) J Am Oil Chem Soc 63(1):29–32
Lee DW, Koh YS, Kim KJ, Kim BC, Choi HJ, Kim DS (1999) FEMS Microbiol Lett 179:393–400
Leow TC, Rahman RN, Basri M, Salleh AB (2007) Extremophiles 11:527–535
Masomian M, Rahman RN, Salleh AB, Basri M (2010) World J Microb Biot 26:1693–1701
Matsumura H, Yamamoto T, Leow TC, Mori T, Salleh AB, Basri M, Inoue T, Kai Y, Rahman RNZRA (2007) Proteins 70(2):592–598
Nawani N, Kaur J (2000) Mol Cell Biochem 206:91–96
Nawani N, Dosanjh NS, Kaur J (1998) Biotechnol Lett 20(10):997–1000
Peng R, Lin JP, Wei DZ (2010) Appl Biochem Biotechnol 162:733–743
Rua ML, Schmidt-Dannert C, Wahl S, Sprauer A, Schmid RD (1997) J Biotechnol 56:89–102
Sinchaikul S, Sookkheo B, Phutrakul S, Pan FM, Chen ST (2001) Protein Expres Purif 22:388–398
Spafiu F, Mischie A, Ionita P, Beteringhe A, Constantinescu T, Balabanb AT (2009) ARKIVOC pp 174–194
Tayyab M, Rashid N, Akhtar M (2011) J Biosci Bioeng 111(3):272–278
Tsujii K (1998) Surface activity: principles, phenomena, and applications. Academic Press, San Diego
Acknowledgments
This research was supported by the Ministry of Science, Technology and Innovation (MOSTI), Malaysia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ali, M.S.M., Yun, C.C., Chor, A.L.T. et al. Purification and Characterisation of an F16L Mutant of a Thermostable Lipase. Protein J 31, 229–237 (2012). https://doi.org/10.1007/s10930-012-9395-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-012-9395-8